
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

April 26, 2024
Written by Marie Diamond and Maria Rizzo Systematic literature reviews (SLR) are essential to informing healthcare...
Read article

April 22, 2024
Defining Probability of Success in Clinical Trial Design with Commercial Software and R Coding
Written by Boaz N. Adler, Director, Global Product Engagement, and J. Kyle Wathen, Vice President, Scientific Strategy...
Read article

April 17, 2024
Early Planning Strategies for External Control Arms in HTA and Regulatory Submissions
Written by Grace Hsu, Evie Merinopoulou, and Jason Simeone To establish treatment efficacy and safety, regulatory and...
Read article

April 15, 2024
Data-Centric Approaches to Streamline the Clinical Workload
In the context of clinical trials, reducing the workload of the clinical team without compromising data quality is...
Read article
April 12, 2024
The New EU HTA Landscape: Insights on Indirect Evidence
How should health technology developers prepare for future market access activities in Europe? Numerous discussions...
Read article

April 10, 2024
Orphan Drug Designation for Rare Diseases
Orphan drug designation is a regulatory status granted to pharmaceuticals developed for the treatment of rare diseases....
Read article

April 8, 2024
Career Perspectives: A Conversation with Joshua Murray
In this latest edition of the Career Perspectives series, we are excited to introduce our readers to Joshua Murray,...
Read article

April 5, 2024
Commercial and Open-Source Software Synergy for Clinical Trial Design
Written by Sydney Ringold, Customer Success Manager, and Kevin Trimm, Chief Product Officer In an ever-changing...
Read article
April 3, 2024
Developing a New Drug Candidate: From Nonclinical to First-in-Human
Thank you to Charlotta Gauffin, Chief Scientific Officer at Dicot, for joining us for our recent webinar, “The Road to...
Read article

April 2, 2024
The FDA’s New Draft Guidance on DMCs: What to Know
Data monitoring committees (DMCs) review ongoing clinical trial data to make recommendations regarding trial conduct...
Read article

March 20, 2024
Key Elements and Implications of the Draft EU JCA Implementing Act
Written by Lydia Vinals, PhD, and Grammati Sarri, PhD The draft Implementing Act of the EU Health Technology Assessment...
Read article

March 13, 2024
Understanding Group Sequential Designs
Group sequential clinical trial designs — a type of adaptive clinical trial design — have emerged as a powerful tool in...
Read article

March 11, 2024
Quantitative Strategies for Rare Disease Clinical Trials
In 2023, rare diseases accounted for 30% of product pipeline under development, about half of which comprising...
Read article

March 4, 2024
Career Perspectives: A Conversation with Arnold van Aswegen
In this new installment of the Career Perspectives series, we had the privilege of interviewing Arnold van Aswegen,...
Read article
February 26, 2024
Sample Size Re-Estimation for Rare Disease Clinical Trials
Written by Boaz N. Adler, MPA, Director, Global Product Engagement, and Valeria Mazzanti, MPH, Associate Director,...
Read article

February 23, 2024
Metadata Repositories: Overcoming Challenges with Automation
Written by Angelo Tinazzi, Nicolas Rouillé, and Sebastià Barceló In the realm of standards management, companies of all...
Read article

February 20, 2024
Negative Binomial Distribution in Group Sequential Designs
In clinical trials based on count data, the aim is to compare independent treatment groups in terms of the rate of...
Read article

February 14, 2024
Erika Spens on Developing Your Regulatory Strategy
During drug development, a comprehensive regulatory strategy is key for saving time and money. There are many common...
Read article

February 13, 2024
Strategies to Overcome Limited Patient Population Challenges in Rare Disease Studies
Written by Boaz N. Adler, MPA, Director, Global Product Engagement, and Valeria Mazzanti, MPH, Associate Director,...
Read article

February 2, 2024
The Living Model Approach for Systematic Literature Reviews
When building a disease model or an economic model, the assumption has been that updates to such models should only...
Read article

January 26, 2024
Parameter Optimization of Multi-Arm Multi-Stage Designs
Innovations in the process of designing adaptive clinical trials have unlocked new possibilities for designing and...
Read article

January 22, 2024
How to Save Time and Limit Costs toward First-in-Human Clinical Trials
Regulatory guidelines outline all crucial studies and documentation that should be in place before a drug product can...
Read article

January 19, 2024
Accelerated DMC Safety Reports for Biotech’s Central Nervous System Studies: A Case Study
Independent data monitoring committees help to ensure patient safety and uphold trial integrity. In Central Nervous...
Read article
January 17, 2024
Late-Stage Clinical Development Strategy: Trade-Offs and Decision-Making in the Confirmatory Setting
Despite accumulating learnings from early phases, several uncertainties remain to be addressed when designing pivotal...
Read article

January 15, 2024
Navigating the Clinical Development Landscape: Insights for Success in 2024
After explosive and frenetic activity in the clinical trial industry during the COVID era, the past two years have seen...
Read article

January 10, 2024
Optimizing Early Clinical Development Strategy
A clinical development strategy is a comprehensive plan designed to establish the safety and efficacy of new...
Read article

January 3, 2024
New FDA Data Submission Requirements and Substantial Changes
Ten years ago this month, in January 2014, the FDA issued the first version of its Technical Conformance Guide (by...
Read article

December 29, 2023
The Top Most-Read Posts of 2023
What a year! Perspectives has explored a myriad of topics this year within clinical development — from adaptive trial...
Read article

December 27, 2023
Top Data Submission and Data Integration Posts of 2023
Perspectives covers a wide range of topics related to data submission and data integration, from ISS and ISE best...
Read article

December 22, 2023
Top Therapeutics Development Topics of 2023
Perspectives covers a wide range of topics within therapeutics development from advice on regulatory submission to...
Read article

December 20, 2023
Top Real-World Evidence and Real-World Data Topics of 2023
Perspectives covers a wide range of topics related to real-world evidence and real-world data, from overcoming health...
Read article

December 15, 2023
The Value of an Optimized Clinical Data Strategy: How Small Changes Can Make a Big Difference
In clinical trials, high-quality data is essential. It drives the drug development decision-making process and is a...
Read article

December 8, 2023
Discussions with the FDA and Ensuring Data Submission Success
Regular technical discussions with the FDA play a critical role in ensuring data submission success. These discussions...
Read article

December 6, 2023
Drug Manufacturer Auditing: Ensuring Quality, Control, and Safety
Chemistry, Manufacturing, and Controls (CMC) is a critical component of drug product development. As a Senior...
Read article

December 4, 2023
Preserving Trial Integrity After Receiving an Unanticipated IDMC Recommendation
Independent data monitoring committees review unblinded clinical trial data and issue recommendations to designated...
Read article

December 1, 2023
Leveraging Real-World Data for Comparison Using Synthetic Control Arms
Last week, Robert Szulkin, Research Principal, Real World Evidence, discussed the need for real-world evidence studies,...
Read article

November 29, 2023
Don’t Forget the Development of Your Placebo: Overcoming Common Obstacles
A clinical trial is usually performed using some kind of comparator. This could be another drug on the market, or a...
Read article

November 24, 2023
Epidemiological Methods to Tackle Real-World Evidence Challenges
Regulatory requirements regarding documentation for new medicines are constantly evolving. Previously, randomized...
Read article

November 20, 2023
Guidelines Are Not Instruction Manuals: Customize Your Way to First-in-Human Clinical Trials
Interpreting all guidelines before your first-in-human clinical trials can be overwhelming. While guidelines are...
Read article

November 17, 2023
FSP Behind the Scenes with Nandan Kothavale, Programming Senior Team Lead
Cytel’s Functional Service Provision (FSP) teams work on exciting projects with biotech and pharmaceutical companies as...
Read article

November 13, 2023
First-in-Human Drug Substance and Formulation: The Challenge of Achieving Flexibility and Quality
For nonclinical studies that precede Phase I, a drug formulation in high doses and concentrations is required. While...
Read article

November 10, 2023
Conduct of IDMCs for Cell and Gene Therapy Trials
Independent data monitoring committees review unblinded clinical trial data and issue recommendations to designated...
Read article

November 8, 2023
Aligning Clinical Trial Design with Investment Priorities
Written by Natalia Muehlemann, Vice President, Clinical Development, and Ari Brettman, Senior Managing Director,...
Read article

November 6, 2023
Ulrika Andersson on First-in-Human Clinical Trial Development
The first-in-human trial, which aims to show the safety and tolerability of a new drug, is a major milestone for any...
Read article

November 3, 2023
Reinforcement Learning: A Promising Tool for Predicting Optimal Treatment in Complex Diseases
Written by Fei Tang and Evie Merinopoulou Reinforcement Learning (RL), a crucial component of machine learning (ML),...
Read article

November 1, 2023
The Changing Landscape of the Pharmaceutical Industry: A Preview of Cytel’s Contributions at PHUSE EU 2023
It feels like just yesterday I attended my first PHUSE conference back in 2005 in Heidelberg, Germany. Fast forward 19...
Read article

October 30, 2023
Managing Uncertainty: Simulation-Based Assurance in Clinical Trial Design
The past two decades have seen the adoption of great innovation in clinical trial design. Statisticians have risen to...
Read article

October 27, 2023
News from ESMO: Challenging the Status Quo of Early Phase Clinical Trial Design — Moving from “Why” to “How”
Written by Natalia Muehlemann, Vice President, Clinical Development; Martin Frenzel, Research Principal, Statistical...
Read article

October 25, 2023
Unravelling PICO: The Pillars of the European Joint Clinical Assessment
The European Union (EU) health technology assessment (HTA) regulation aims to improve the availability of innovative...
Read article

October 23, 2023
Experiencing the CBER: Anticipating Unique Challenges
The Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) are the...
Read article
October 20, 2023
Mind the Health Gap: Is It Time to Reliably Measure the Impact of Health Inequity in Product Development and Assessment? Yes, It Is.
Written by Grammati Sarri and Yannis Jemiai The spotlight for this year’s World Evidence-Based Healthcare Day (EBHC)...
Read article
October 18, 2023
Preparing Your Integrated Summaries of Safety and Effectiveness: Best Practices
Written by Angelo Tinazzi and Florence Le Maulf Integrated Summaries of Safety (ISS) and Integrated Summaries of...
Read article

October 13, 2023
Looking to the Future — Improving Diagnosis and Prognosis of Eye Conditions with Artificial Intelligence
Written by Alind Gupta, Cytel; Haridarshan Patel, Horizon Therapeutics; and Jason Simeone, Cytel Ophthalmology is...
Read article

October 11, 2023
Global Planning to Local Execution Market Success
Moving beyond static evidence development to ensure local market access success; responding to recent changes in...
Read article

October 10, 2023
FSP Behind the Scenes with Brooke Smith, Senior Biostatistician
Cytel’s Functional Service Provision (FSP) teams work on exciting projects with biotech and pharmaceutical companies as...
Read article

October 6, 2023
Writing a Successful Study Protocol for Real-World Evidence Studies
Real-world evidence studies are becoming increasingly popular in pharmaceutical development. But to ensure such studies...
Read article

October 4, 2023
How to Ensure Your Adaptive Trial Is Appropriate for Regulatory Submission
Adaptive clinical trial designs have become increasingly popular among developers and investors due to the many...
Read article

October 2, 2023
A Better Way to Track Medication Adherence
Patients’ adherence to the medications or treatment regimens prescribed to them by their clinicians is an important...
Read article

September 29, 2023
Innovative Clinical Trial Design: Commercial vs Open-Source Software? Why Not Both?
In the ever-changing field of clinical trial design, there is often a need to evaluate design options quickly and...
Read article

September 25, 2023
Reducing Independent Data Monitoring Committee Timelines: A Focus on Formal Interim Analyses
As the pressure to reduce timelines rises across the industry, independent data monitoring committees (IDMCs) — which...
Read article

September 22, 2023
Myth Busting: Master Protocol Edition
Interest and appetite for master protocols is growing as sponsors consider opportunities in various therapeutic areas...
Read article

September 20, 2023
FSP Behind the Scenes with Anwaya Joshi, Programming Senior Team Lead
Cytel’s Functional Service Provision (FSP) teams work on exciting projects with biotech and pharmaceutical companies as...
Read article

September 18, 2023
Cytel Publishes Inaugural Sustainability Report
With the ongoing changes inherent in the life sciences industry comes heightened expectations for companies to take...
Read article

September 13, 2023
Setting Expectations for Formal Interim Analyses with Independent Data Monitoring Committees
Independent data monitoring committees (IDMCs) review ongoing clinical trial data to make recommendations regarding...
Read article

September 11, 2023
How to Ask the Right Questions at an Authority Meeting
As a drug developer, you have to live with the answers and comments you get from regulatory authorities. Therefore,...
Read article

September 8, 2023
Synthetic Control Arms: Leveraging Real-World Data for Comparison in Single-Arm Trials
Single-arm trials, unlike placebo-controlled randomized control trials, forgo the use of a placebo or standard-of-care...
Read article

September 1, 2023
Key Considerations in Planning Your Clinical Data Strategy
Data is the cornerstone of any clinical trial, driving the decision-making process of drug development, and is a...
Read article

August 30, 2023
FSP Behind the Scenes with Jeff Thompson, R Senior Statistical Programmer
Cytel’s Functional Service Provision (FSP) teams work on exciting projects with biotech and pharmaceutical companies as...
Read article

August 28, 2023
How to Create and Optimize a Clinical Development Plan
A clinical development plan — a comprehensive strategy for developing an investigational product through regulatory...
Read article

August 25, 2023
Preparing and Concluding Your FDA Data Submission, and More Insights on Data Submission and Data Integration
For several years, CDISC and Regulatory Data Submission expert Angelo Tinazzi has authored the series, The Good Data...
Read article

August 22, 2023
Adaptive Multi-Arm Multi-Stage Designs: A Comparison of Methods
Written by Cyrus Mehta and Heather Struntz The significant time and cost, as well as high failure rates, of clinical...
Read article

August 18, 2023
The Advantages of Forecasting Enrollment with a Model-Based Approach
The most common cause for incomplete Phase III trials is enrollment. Indeed, as many as 37% of trials miss...
Read article

August 14, 2023
The Evolution of Open-Source Initiatives and New Standards Development for the Data Submission of the Future
In the first part of this post, I discussed the ongoing revolution, or maybe I should say evolution, we are living...
Read article

August 11, 2023
Demonstrating Value in Real Time with Living Models for Systematic Literature Reviews
Systematic literature reviews are essential for proving product value to health authorities, clinicians, and payers,...
Read article

August 9, 2023
Standards and Open Source Hand-in-Hand: Leveraging Automation to Expedite Drug Market Request Review Process
How do you envision the future of data submission? Last week, I had the privilege of presenting the topic “Standards...
Read article

August 4, 2023
Bayesian Methods for Strategic Clinical Trial Design
“There is always the risk that interim analyses might occur after the Sufficient Information Threshold has been...
Read article

August 1, 2023
Dynamic Bayesian Borrowing to Bolster Limited Sample Sizes in Rare Indications
Evaluating the efficacy and safety of novel therapies in rare indications can be challenging due to the difficulty of...
Read article

July 28, 2023
Embracing AI and ML in Medical Devices: FDA’s Total Product Lifecycle-Based Regulatory Framework
Written by Fei Tang, RWE Senior Research Consultant, and Paul Arora, Assistant Professor (Status), Dalla Lana School of...
Read article

July 14, 2023
Real-Life Data-Sharing and EU Joint Clinical Assessments: Is Closing this Chasm a Mission Impossible?
Written by Grammati Sarri, David Smalbrugge, Andreas Freitag, and Evie Merinopoulou The vision of a single, centralized...
Read article


June 20, 2023
New FDA Guidelines on Pediatric Studies and Potential Effects on Opportunities for Market Exclusivity
Legislation on pediatric studies has existed for more than 20 years in the US, yet additional guidance from the FDA has...
Read article

June 14, 2023
Embracing Innovation: Exploring the Design of Umbrella and Basket Trials
Medical research has come a long way in recent years, fueled by innovative trial designs that challenge traditional...
Read article

June 9, 2023
Maximizing Study Momentum: A Case Study in Accelerated DMC Safety Report Creation through IDMC Solutions
In the ever-evolving landscape of clinical development, the need for robust evaluation of interim clinical data through...
Read article

June 7, 2023
A Spotlight on FSP Programming
Since its founding in 1987, Cytel has been home to some of the most passionate, creative, and talented experts in the...
Read article

June 5, 2023
Maria Lundberg on a Holistic Approach to Therapeutics Development
Cytel recently announced the launch of its Therapeutics Development Team, bringing together quantitative,...
Read article

June 2, 2023
Presenting Clinical Data for Regulatory Submission: A Stats Perspective
Data submissions are very regulated, but every drug and drug development are different. Therefore, the data presented...
Read article

May 24, 2023
It’s Time to Move, Time to Move to Define-XML 2.1
As of March 2023, specifically for any study started on or after March 15, 2023,1 for the submission of SEND, SDTM, and...
Read article

May 22, 2023
New CADTH Guidance on RWE Is Now Available, but Critical Aspects Are Still Missing
By Grammati Sarri, Evie Merinopoulou, Vinusha Kalatharan, and Jason Simeone The Canadian Agency for Drugs and...
Read article


May 10, 2023
FDA Increases Calls for Manufacturers to Ensure Trial Diversity, but Does It Fall Short of Addressing Health Inequalities in Product Development?
The evidence is staggering on the unequal health burdens experienced by specific patient groups defined by ethnic,...
Read article

May 5, 2023
Scott Gaines on the Power of Simulation-Guided Design to Handle Increasingly Complex Clinical Trials
As clinical trials become more complex, simulation-guided design approaches are crucial. For this edition of the...
Read article

May 3, 2023
Reflections on the RCT DUPLICATE Study and Increasing Confidence in Real-World Evidence
With input by Alind Gupta, Louis Dron, and Jason Simeone. Randomized clinical trials (RCTs) have long been considered...
Read article

April 26, 2023
New Ebook: “The Good Data Doctor on Data Submission and Data Integration”
Regular technical discussions with the FDA play a critical role in ensuring data submission success. These discussions...
Read article

April 25, 2023
How Target Trial Emulation Can Take the Guesswork Out of Comparative Effect Estimates in Medicare Drug Price Negotiation
An interview with Miguel Hernán, Harvard University Kolokotrones Professor of Biostatistics and Epidemiology On March...
Read article

April 24, 2023
APAC Biopharma Industry Insights: Trends, Opportunities, and Challenges
In the last 10 years, the Asia-Pacific (APAC) region has become a hotspot for clinical trials: the region contributed...
Read article

April 21, 2023
CDISC Europe 2023: A Preview
It was early March 2020, after the world was hit by the Covid-19 pandemic, that those of us on the CDISC Eu committee...
Read article

April 18, 2023
Navigating Comparative Effectiveness in the Inflation Reduction Act: Methodological Approaches for Healthcare Challenges
The Inflation Reduction Act (IRA), passed in August 2022, marks a significant shift in the US healthcare landscape,...
Read article

April 14, 2023
Data Challenges (and Solutions) for Externally Controlled Trials
Real-world data and evidence are increasingly being used in health care decisions and publications. However, there are...
Read article

April 11, 2023
Overcoming the Shared Effect Modifier Assumption with Network Meta-Interpolation
Commonly used methods to handle the complexities of effect modification in indirect treatment comparisons (ITCs) often...
Read article
April 5, 2023
Comparative Effectiveness: Methods and Techniques for Better Decision-Making
Health technology assessment (HTA) submissions require cost effectiveness analyses based on comparative effectiveness...
Read article

March 24, 2023
Winter Weekend Read Roundup
Last week, we featured our final Winter Weekend Read, the last in a series designed to showcase our complimentary...
Read article

March 21, 2023
Retrospective Claims Data Analysis Unlocks Discovery in Multiple Sclerosis Research
One of the lesser-known complications associated with Multiple Sclerosis is a higher risk of serious infections (SIs)....
Read article

March 17, 2023
Optimizing Small Clinical Trials with Simulation-Guided Design: A Case Study
Smaller clinical trials can be optimized in significant ways using simulation-guided design. A small biotech studying...
Read article

March 13, 2023
Industry Voices: Yannis Jemiai on Simulation-Guided Design and the Changing Landscape of Clinical Trial Strategy
Many industries have long since adopted the practice of modeling and simulating experimental scenarios. And despite...
Read article
March 8, 2023
New Linked Database Expands Potential of RWE: A Unique Opportunity for Multiple Sclerosis Research
Real-world data has been increasingly used to answer questions related to the course, prognosis, and treatment of...
Read article

March 7, 2023
Can RWE Help Restore Decades of Health Inequalities? Yes, and Here’s How
Health inequalities are an enduring issue that can be exacerbated by clinical trial recruitment that does not reflect...
Read article

March 3, 2023
Multiple Myeloma Research Benefits from Living Model SLR: A Case Study
The speed of scientific discovery has been outpacing the ability of researchers to accumulate and integrate constantly...
Read article

February 24, 2023
Demystifying Synthetic Control Arms
Last week, Cytel Director & Research Principal Louis Dron discussed new FDA guidance on the design and conduct of...
Read article

February 22, 2023
Simulation-Guided Design for Biotechs
Simulation-guided design is quickly becoming a novel feature of modern drug development. Its foundational promise is to...
Read article

February 14, 2023
FDA Guidance on the Design and Conduct of Externally Controlled Trials — What to Watch
The U.S. FDA has recently provided specific guidance[i] on the design and conduct of trials incorporating an external...
Read article

February 9, 2023
Supercharging Quantitative Decision-Making with Simulation-Guided Trial Design
Those familiar with simulation-guided design (SGD) know that it can be used for a wealth of clinical trial options:...
Read article

February 8, 2023
The Facts in the Case of Subject X
Over the past years, probably the entire last decade, there have been several discussions on how to handle multiple...
Read article

February 3, 2023
Focal Points and Monetization: New Uses of Pareto Frontiers in Clinical Development
For clinical development and research and development teams, the Pareto Frontier can perform two functions. Let’s take...
Read article

January 31, 2023
Linked Data Studies: Improving the Way We Do Observational Research in Germany
Asthma affects more than 235 million people worldwide, and due to lacking effective implementation of clinical...
Read article

January 27, 2023
To Adapt or Not to Adapt? A Decision Framework
Should your clinical trial be adaptive? Trials that include a prospectively planned modification based on an interim...
Read article

January 25, 2023
Bayesian Approach in Oncology Trials
People think in Bayesian terms all the time: we use prior information and the evidence at hand to make decisions in our...
Read article

January 23, 2023
Design Considerations for Early Phase Trials of Immuno-oncology Drugs
Ever since the first immune checkpoint inhibitor was approved for market nearly twelve years ago, the industry has...
Read article

January 20, 2023
Strategic Clinical Trial Design Unlocks Innovative Funding Opportunity
A small biotech’s conventional carcinoma trial design was too expensive to implement and did not offer the opportunity...
Read article

January 17, 2023
Bayesian Strategies in Rare Diseases
When it comes to rare diseases, a handful of major challenges to drug development arise. Bayesians strategies have...
Read article

January 13, 2023
Bayesian Methods across the Clinical Development Journey
Bayesian methods, with their ability to facilitate flexibility and learning, are often associated with early-phase...
Read article

January 12, 2023
Why Are There Not More Bayesian Clinical Trials?
Statistical methods have long been fundamental to drug development, and advancements in the last few decades in...
Read article

January 6, 2023
Simulation-Guided Design Is Reshaping Clinical Trial Strategy
You may have heard that our clinical trial strategy platform Solara® won the Fierce Life Sciences award for Technology...
Read article

January 4, 2023
A Look Ahead for 2023
Returning to Cytel after the winter holidays, I am excited to begin a year that will likely prove memorable for both my...
Read article

December 29, 2022
Top Perspectives Articles of 2022
Perspectives on Enquiry and Evidence explores a wide variety of topics within clinical trial design and data science in...
Read article

December 27, 2022
Top Bayesian Topics of 2022
Bayesian methods have been playing a key role in transforming clinical research, providing a variety of new...
Read article

December 21, 2022
Top Interviews of 2022: Industry Voices and Career Perspectives
Perspectives on Enquiry and Evidence features two recurring interview series: Our new Industry Voices series, in which...
Read article

December 19, 2022
Top Adaptive Clinical Trial Topics of 2022
Adaptive trial designs – that is, trials that include a prospectively planned modification based on an interim analysis...
Read article

December 16, 2022
Topics in Bayesian Statistical Methods
Bayesian methods have been playing a key role in transforming clinical research, and Bayesian topics are frequently...
Read article

December 14, 2022
Accrual When Starting a Platform Trial vs. in a Stand-Alone Trial
When evaluating the efficacy of a candidate investigational therapy, a standard clinical trial paradigm is to conduct a...
Read article

December 9, 2022
(Re)Integration Dilemma: Integrated Summaries of Safety and Effectiveness
As promised in my last post prior to PHUSE-EU Connect, I’d like to now share some reflections on my “Integration...
Read article

December 6, 2022
Career Perspectives: A Conversation with Veronica Chan
In this edition of the Career Perspectives series, I spoke with Veronica Chan, Principal Clinical Data Manager at...
Read article

December 2, 2022
Celebrating 35 Years of Innovation and Impact: An Interview Series
For 35 years, Cytel’s scientific rigor and operational excellence have enabled biotech and pharmaceutical companies to...
Read article

November 29, 2022
Bayesian Adaptive Clinical Trial Designs: INLA vs. MCMC
Bayesian methods have continuously played a key role in transforming clinical research in therapeutic areas such as...
Read article

November 18, 2022
Industry Voices: Dr. Parvin Fardipour on New Horizons in Data Science
In the following interview, Dr. Parvin Fardipour, Quantitative Strategies & Data Science, sits down with Heather...
Read article

November 14, 2022
Network Meta-Interpolation: Effect Modification Adjustment in Network Meta-Analysis Using Subgroup Analyses
When conducting network meta-analysis (NMA) – that is, a technique that involves comparing multiple treatments...
Read article

November 9, 2022
Career Perspectives: A Conversation with Malte Stein
In this edition of the Career Perspectives series, I interview Malte Stein, Senior Biostatistician. Malte owned the...
Read article

November 8, 2022
A Preview of Cytel’s Contributions to PHUSE EU 2022
Although many of you can’t wait for the start of the Football World Cup 2022 (less than two weeks while I’m writing),...
Read article

November 3, 2022
Cytel at ISPOR Europe: Two Workshops
Cytel will be represented at over 60 presentations at ISPOR Europe 2022, with more issue panels and workshops than any...
Read article

November 2, 2022
Bayesian Hierarchical Modelling for Histology-Independent Therapies
Pharmaceutical research in oncology is increasingly focused on the development of therapies targeted at newly...
Read article

October 28, 2022
Measuring Robustness of Clinical Trial Designs with Pressure Tests
Integrating the “pressure testing” of clinical trial designs into the process of creating a strong clinical trial...
Read article


October 24, 2022
Cyrus Mehta on the Founding of Cytel
On the occasion of Cytel’s 35th anniversary, co-founder Professor Cyrus Mehta sits down with Dr. Esha Senchaudhuri to...
Read article



October 18, 2022
Nitin Patel on 35 Years of Technological Innovation
On the occasion of Cytel’s 35th anniversary, co-founder Professor Nitin Patel sits down with Dr. Esha Senchaudhuri to...
Read article

October 14, 2022
Career Perspectives: A Conversation with Nicolas Rouillé
In this edition of the Career Perspectives series, I interview Nicolas Rouillé, Senior Director, Statistical...
Read article

October 13, 2022
Cytel at ISPOR Europe: Top Presenter of Issue Panels and Workshops
ISPOR Europe, the leading global conference for health economics and outcomes research (HEOR) and real-world evidence...
Read article

October 11, 2022
Joshua Schultz on the Evolution of Cytel
On the occasion of Cytel’s 35th anniversary, our CEO Joshua Schultz sits down with Dr. Esha Senchaudhuri to discuss the...
Read article

October 5, 2022
Platform Trials, Can they Benefit Animal Studies?
Master protocols and platform clinical trials have become an innovative and efficient approach to testing multiple...
Read article

October 4, 2022
MCMC vs. INLA in Bayesian Adaptive Clinical Trial Designs
Integrated Nested Laplacian Approximations (or INLA) are now starting to be used by statisticians as a key tool for...
Read article

October 3, 2022
Bayesian Methods for Historical Borrowing: Conjugate Priors
The wider availability of electronic health data, medical registries, and even larger proprietary datasets means that...
Read article


September 28, 2022
Statistical Leaders and the Future of Drug Development
The landscape of drug development has changed dramatically over the last few decades, and effective statistical leaders...
Read article

September 27, 2022
Data Capture and Data Sharing During the COVID-19 Pandemic
On Louis Dron et al., “Data Capture and Sharing in the COVID-19 Pandemic: A Cause for Concern,” The Lancet 4 (10) (2022)
Read article

September 23, 2022
Adaptive Trials at the Mainstream of Drug Development
Adaptive trial designs – that is, trials that include a prospectively planned modification based on an interim analysis...
Read article

September 16, 2022
Raising Awareness for Additional FDA Data Standards Submission Recommendations (Part II)
In the first part of this article, I raised awareness of the availability of additional FDA guidances containing CDISC...
Read article

September 15, 2022
U.S. Drug Pricing Reform: Potential Impact on Pharma HEOR Evidence Generation
On August 16, 2022, President Biden signed into law the Inflation Reduction Act of 2022, which includes U.S. drug...
Read article

September 12, 2022
On Frequentist and Bayesian Sequential Clinical Trial Designs
In clinical trials, patient enrollment is often staggered, with data collected sequentially. When designing a clinical...
Read article

September 9, 2022
Developing Synthetic Control Arms Using Bayesian Models
A new trend has emerged over the last decade that has changed the way many clinical trials are conducted. Unlike...
Read article

September 8, 2022
Cytel Present at the ASA Biopharmaceutical Section Regulatory Workshop
The American Statistical Association Biopharmaceutical Section, in cooperation with the FDA Statistical Association,...
Read article

September 2, 2022
Summer Weekend Read Roundup
Last week, we featured our final Summer Weekend Read, the last in a series designed to showcase some of our most recent...
Read article

August 30, 2022
Understanding the Economic Benefits of Platform Trials
Many thanks to Kyle Wathen and Behnam Sharif for their input on this post.
Read article

August 26, 2022
Discover the Value of an Optimized Clinical Data Strategy
To continue our Summer Weekend Reads series, Cytel presents “Discover the Value of an Optimized Clinical Data Strategy”...
Read article

August 25, 2022
Cytel Presents at ICPE 2022
The International Society for Pharmacoepidemiology is hosting its “ICPE 2022: Advancing Pharmacoepidemiology and...
Read article


August 18, 2022
Highlights from JSM 2022
The American Statistical Association’s annual Joint Statistical Meeting (JSM) gathered over 6,500 attendees from 52...
Read article




August 4, 2022
Career Perspectives: Interview with Allison Luccock
In this edition of the Career Perspectives series, I interview Allison Luccock, Director of Business Operations for...
Read article

August 3, 2022
The Uses of Bayesian Methods in Late-Phase Clinical Trial Strategy
A number of late-phase clinical trial sponsors remain hesitant to employ Bayesian approaches in confirmatory settings,...
Read article


July 28, 2022
New Directions in Indirect Treatment Comparisons
When new treatments are compared with existing therapies in clinical care, population-adjustment techniques need to...
Read article

July 27, 2022
Adaptive Designs Are Re-Defining Drug Development – Learn What's New
Written by Jing Ping Yeo and Charles Warne Adaptive designs are studies that “include a prospectively planned...
Read article

July 26, 2022
7 Ways RWD Is Transforming Clinical Research
To watch this webinar and others from this introductory series, click the link below. The ability to draw on electronic...
Read article


July 20, 2022
The Case for Network Meta-Interpolation to Handle Effect Modifiers in Indirect Treatment Comparisons
When performing indirect treatment comparisons, effect modification can create complexities in the event of high...
Read article


July 13, 2022
5 Steps to Adjust for Effect Modifiers for Treatment Comparisons
Many thanks to Grammati Sarri and Michael Groff for their comments in developing this blog. An indirect treatment...
Read article

July 12, 2022
Strategies for Selecting New Indications for a Platform Trial
Thanks to Dr. Kyle Wathen for comments on this blog. The increasing use of platform trials for the testing of a wide...
Read article

July 7, 2022
Using Quantitative Bias Analysis in Real World Data Strategy
The gold standard for assessing the efficacy for a medicine continues to be RCTs, however, for many reasons (disease...
Read article

June 29, 2022
Platform Trials, Master Protocols, and Challenges in Execution
How can we build an efficient statistical protocol for a clinical trial, if we do not know the therapies that will be...
Read article

June 28, 2022
Career Perspectives: Reflecting at 10 Years in Cytel FSP
Founded in 1987 by Cyrus Mehta and Nitin Patel, research scientists at Harvard University and MIT respectively and...
Read article

June 22, 2022
How to Determine if Your Clinical Trial Has Sufficient Data?
It can be difficult to estimate just how much time and data you need to address the multitude of considerations that...
Read article

June 15, 2022
Raising Awareness for FDA Data Submission Recommendations (I)
For years CDISC data standards implementers have struggled to find good implementation examples and use cases beside...
Read article
June 14, 2022
6 Key Trials for Understanding Adaptive Designs for Clinical Trials
Suppose you had to choose six clinical trials intended for registration with regulatory agencies, only six, to explain...
Read article

June 13, 2022
Driving Global Data Collaboration for COVID-19
The International COVID-19 Data Alliance (ICODA) was formed to address the challenge of generating rapid and rigorous...
Read article
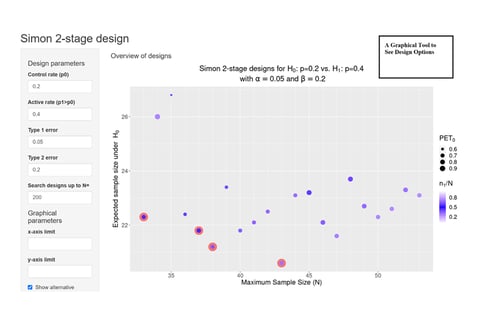
June 8, 2022
On Kappler's 'Graphical Comparison of Simon two-stage designs'
Clinical researchers, seeking to understand the statistical benefits of a common Phase 2 oncology design, now have a...
Read article
June 7, 2022
Continuous Monitoring for Blinded Sample Size Reestimation
In most instances of blinded sample size re-estimation, the timing of the interim analysis that determines whether the...
Read article

June 6, 2022
The TOGETHER Trial Journey: Interview with Ofir Harari
The award-winning TOGETHER Trial was designed with the vision of ensuring that COVID-19 therapies are both effective...
Read article

June 2, 2022
Digital Transformation for Clinical Trials
How can clinicians at the forefront of modern clinical trials and statisticians at the forefront of advanced...
Read article

May 31, 2022
What it means to be a lead analyst on a Global COVID-19 Trial
The TOGETHER Trial for COVID-19 therapies, designed by clinical trial specialists at Cytel won the Society for Clinical...
Read article

May 27, 2022
How to Use a Living HTA Approach to Demonstrate Value in Real-Time
When submitting systematic literature reviews to a Health Technology Assessment authority, high volumes of research...
Read article

May 26, 2022
Method of Estimation with application to the COVID-19 Pandemic
When constructing estimands a key question that arises is how to handle intercurrent events and missing data. In a...
Read article

May 24, 2022
Leveraging Advanced Statistical Software to Optimize Clinical Development
Traditionally, clinical trials are expensive, long in duration, and have low success rate. But with the advent of rich...
Read article
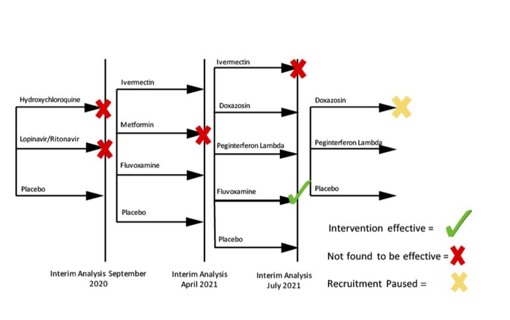
May 17, 2022
Society of Clinical Trials names TOGETHER "Trial of the Year"
Early in the pandemic, it became clear that many of the COVID-19 therapies being tested in wealthier nations, were not...
Read article

May 16, 2022
Cytel & ARCS Collaborate to Strengthen Early Phase Capabilities
A combination of industry and policy forces have recently changed the shape of Australia’s R&D sector, making it a...
Read article

May 12, 2022
Estimator Choice in Synthetic Control Arm Analyses
Synthetic control arm (SCA) methods are statistical methods that are seeing rapidly increasing use in comparative...
Read article

May 5, 2022
Interview with Grammati Sarri on Moderating an Issue Panel 2022
While randomized control trials remain the industry gold-standard for regulatory and reimbursement submissions, there...
Read article

May 4, 2022
Optimizing Human-Machine Partnership for Up-To-Date Evidence
If you are a pharmaceutical or biotech company seeking to enter the market with a new drug, you need to submit a...
Read article


