How to Create and Optimize a Clinical Development Plan
A clinical development plan — a comprehensive strategy for developing an investigational product through regulatory submission — is a critical component of drug development and helps ensure that new therapies are safe, effective, and of high quality. Here, I offer a high-level overview on steps to take to create a clinical development plan, how to optimize your plan as the program progresses, and why this comprehensive roadmap is so vital to the success of your development program.
How to create a clinical development plan
Creating a clinical development plan typically involves a multidisciplinary team that includes experts in clinical development, regulatory affairs, medical affairs, statistics, and other relevant areas. Consider the following steps when creating a clinical development plan:
-
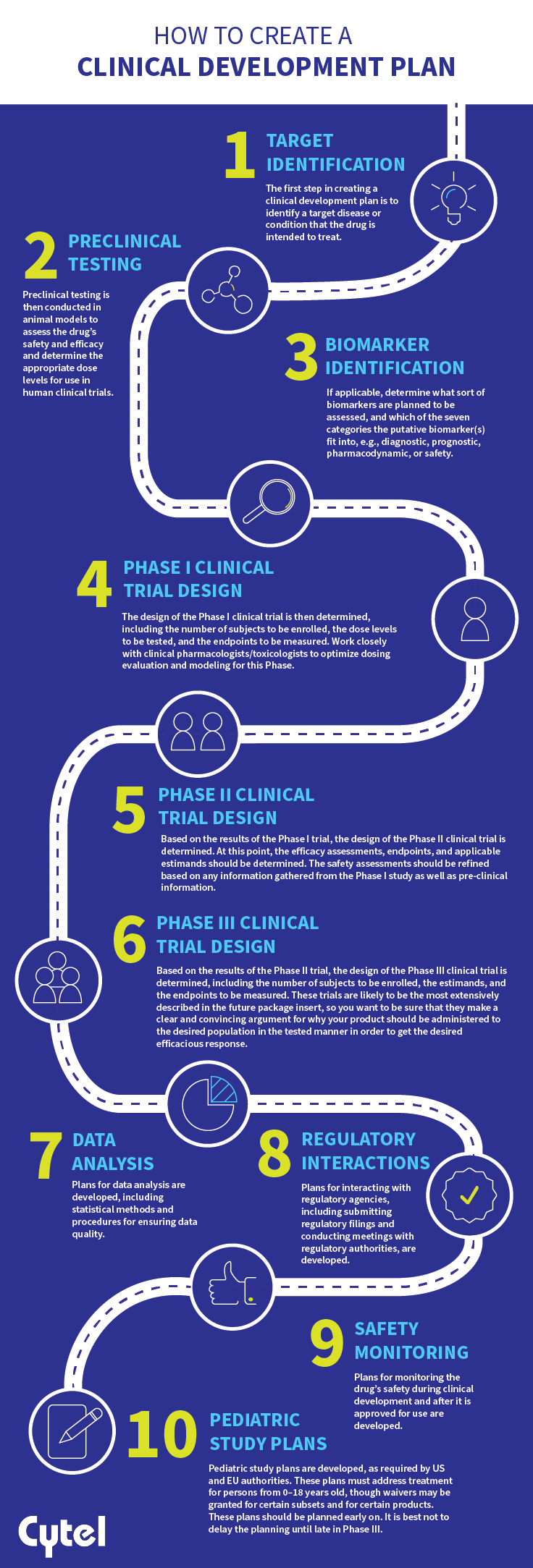
- Target identification: The first step in creating a clinical development plan is to identify a target disease or condition that the drug is intended to treat.
- Preclinical testing: Preclinical testing is then conducted in animal models to assess the drug’s safety and efficacy and determine the appropriate dose levels for use in human clinical trials.
- Biomarker identification (if applicable): Determine what sort of biomarkers are planned to be assessed, and which of the seven categories the putative biomarker(s) fit into, e.g., diagnostic, prognostic, pharmacodynamic, or safety.
- Phase I clinical trial design: The design of the Phase I clinical trial is then determined, including the number of subjects to be enrolled, the dose levels to be tested, and the endpoints to be measured. Work closely with clinical pharmacologists/toxicologists to optimize dosing evaluation and modeling for this Phase.
- Phase II clinical trial design: Based on the results of the Phase I trial, the design of the Phase II clinical trial is determined. At this point, the efficacy assessments, endpoints, and applicable estimands should be determined. The safety assessments should be refined based on any information gathered from the Phase I study as well as pre-clinical information.
- Phase III clinical trial design: Based on the results of the Phase II trial, the design of the Phase III clinical trial is determined, including the number of subjects to be enrolled, the estimands, and the endpoints to be measured. These trials are likely to be the most extensively described in the future package insert, so you want to be sure that they make a clear and convincing argument for why your product should be administered to the desired population in the tested manner in order to get the desired efficacious response.
- Data analysis: Plans for data analysis are developed, including statistical methods and procedures for ensuring data quality.
- Regulatory interactions: Plans for interacting with regulatory agencies, including submitting regulatory filings and conducting meetings with regulatory authorities, are developed.
- Safety monitoring: Plans for monitoring the drug’s safety during clinical development and after it is approved for use are developed.
- Pediatric study plans are developed, as required by US and EU authorities. These plans must address treatment for persons from 0–18 years old, though waivers may be granted for certain subsets and for certain products. These plans should be planned early on. It is best not to delay the planning until late in Phase III.
- Target identification: The first step in creating a clinical development plan is to identify a target disease or condition that the drug is intended to treat.
The clinical development plan is then reviewed and revised as needed to ensure that it meets regulatory requirements, addresses safety concerns, and provides a clear roadmap for the clinical development of the drug. The plan is a living document, updated regularly as new data becomes available and the development program progresses.
How to optimize your clinical development plan
Optimizing a clinical development plan involves making adjustments to ensure that the development program is designed to achieve the best possible outcome while minimizing risks and costs. It will involve expert input, careful consideration of the available data, and a willingness to adjust the plan as needed. Consider the following steps:
-
- Conduct a thorough review: The first step to optimize a clinical development plan is to thoroughly review the existing plan, including the available data and the regulatory landscape. This will help identify areas that need improvement or adjustment.
-
- Consult with experts: Consult with experts in relevant areas, including clinical development, regulatory affairs, medical affairs, and statistics, to obtain their input and recommendations.
-
- Refine the target population: Refine the target population by conducting additional analysis of patient demographics, disease characteristics, and other relevant factors to identify subgroups of patients that may benefit most from the drug.
-
- Optimize the trial design: Optimize the trial design by adjusting the sample size, the number of study arms, and the selection of endpoints to increase the chances of demonstrating efficacy and safety.
-
- Use adaptive trial designs: Consider using adaptive trial designs that allow for changes in the trial design based on interim data analysis to maximize efficiency and flexibility.
-
- Incorporate biomarkers: Incorporate biomarkers into the trial design to identify patients most likely to respond to the drug and to monitor the drug’s efficacy and safety.
-
- Use real-world evidence (RWE): Consider incorporating RWE to optimize the study design, identify new patient populations, and enhance understanding of the drug’s real-world effectiveness.
-
- Optimize the regulatory strategy: Work with regulatory agencies to optimize the regulatory strategy, including selecting endpoints and developing a compelling benefit-risk profile.
- Optimize the regulatory strategy: Work with regulatory agencies to optimize the regulatory strategy, including selecting endpoints and developing a compelling benefit-risk profile.
The importance of the clinical development plan
A clinical development plan is an essential component of drug development because it provides a comprehensive roadmap for the clinical trials required to demonstrate the safety and efficacy of a new drug or therapy. A thorough clinical development plan will:
-
- Ensure safety: A clinical development plan outlines the steps required to assess the drug’s safety in humans. By conducting clinical trials in a structured and systematic manner, potential safety issues can be identified early in the development process, allowing appropriate steps to be taken to address them.
-
- Demonstrate efficacy: The clinical development plan outlines the steps required to demonstrate the efficacy of the drug in treating the target disease or condition. By conducting clinical trials in a structured and systematic manner, the drug’s effectiveness can be accurately assessed, and potential issues can be identified and addressed.
-
- Provide a regulatory roadmap: The clinical development plan outlines the steps required to obtain regulatory approval for the drug. By adhering to the plan, developers can ensure they meet the regulatory requirements for drug development, which can help speed up the approval process.
-
- Help manage resources: The clinical development plan provides a roadmap for allocating resources, including time, money, and personnel. By having a clear plan, developers can allocate resources more efficiently, reducing costs and maximizing the chances of success.
At Cytel, our Therapeutic Development Team comprises experts who can support your team throughout the development lifecycle. Click the button below to speak with us about how we can help you create, refine, and optimize your clinical development plan to demonstrate the safety and efficacy of your drug, ensure compliance with regulatory requirements, and help manage your investment effectively.
Read more from Perspectives on Enquiry and Evidence:
Sorry no results please clear the filters and try again

New FDA Guidelines on Pediatric Studies and Potential Effects on Opportunities for Market Exclusivity

Adaptive Trials at the Mainstream of Drug Development

Real-Life Data-Sharing and EU Joint Clinical Assessments: Is Closing this Chasm a Mission Impossible?


