Mind the Health Gap: Is It Time to Reliably Measure the Impact of Health Inequity in Product Development and Assessment? Yes, It Is.
Written by Grammati Sarri and Yannis Jemiai
The spotlight for this year’s World Evidence-Based Healthcare Day (EBHC) (October 20, 2023), is on the generation and application of evidence to improve global health equity.
According to the World Health Organization (WHO), “health equity is the absence of unfair, avoidable or remediable differences in health among population groups, defined by social, economic, demographic or geographic characteristics.”
Health inequity (or disparity) is not a new health topic; it has been widely documented across the world for the last four decades. What is new are the recent efforts to make health equity a strategic priority in healthcare decision-making. A couple of things are clearer now more than ever — 1) there is a need to generate reliable data to measure health inequities, and 2) we imperatively must shift from passive consumption of this information in the form of purely epidemiological study findings and/or public prevention efforts to a pro-active structured methodological effort to quantify the impact of these inequities on health outcomes. Consequently, the key questions for those involved in health product development are the following:
- First of all, is there sound epidemiological background evidence to quantify existing health inequities (disparities) for the patient population targeted by the new product? If yes, then:
- How can evidence generation (trial recruitment, additional evidence collected through real-world studies) ensure that the clinical profile of this new product reflects the experiences of a diverse patient group? How can this inclusive data collection approach be implemented by overcoming historical access barriers and how can it be standardized across technologies?
- How can researchers reliably differentiate the value of a new product across different patient groups? How much weight should decision-makers give to a new product that closes health inequity gaps (equity decision-making criteria)? Is assigning innovation weight to decreasing health inequities the standardized way forward for decision-makers?
Achieving health equity is multifaceted, politically determined, and rooted in different sociocultural historical backgrounds. Thus far, health equity remains left out of consideration for health product development and assessment mainly driven by a lack of explicit requirements on this issue by regulators and payers.
Recently, decision-making frameworks in the healthcare setting are evolving. We have seen increased recognition by health decision-makers and international organizations of the urgent need to address evidence-based health inequities during the product development cycle. This has resulted in many guidance documents from regulatory agencies and payers around the world.
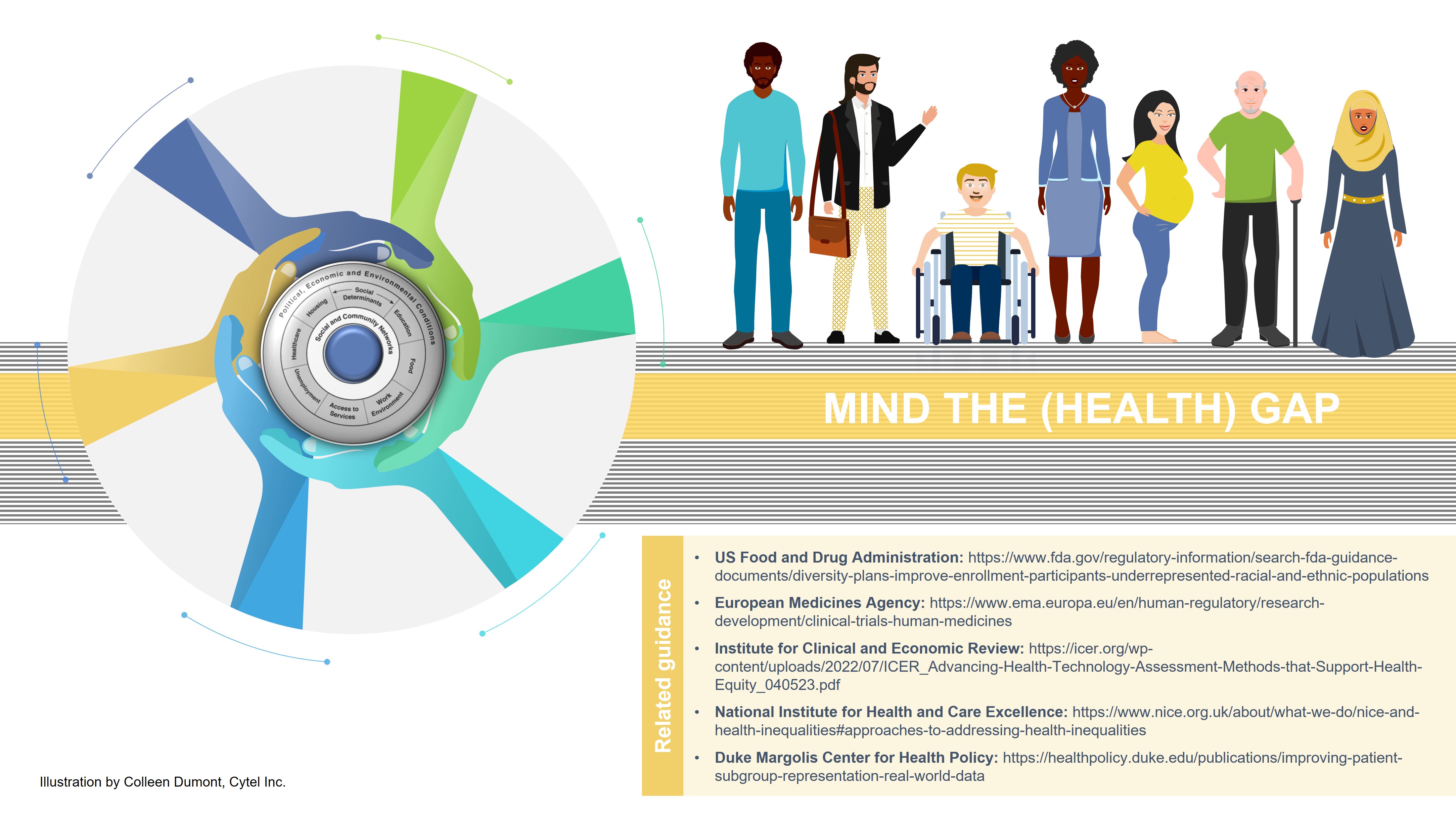
For example, diversity, equity, and inclusion in trial development planning and enrollment practices are at the forefront of recent guidance from the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA); this guidance is putting pressure on drug sponsors to ensure patients from underrepresented racial and ethnic groups are equally considered in clinical trials. Clinical trials often have been subject to persistent issues of inequity and inequality, raising significant ethical, social, and economic alarms. Certain populations, such as racial and ethnic minorities, women, the elderly, people with disabilities, and individuals from low-income backgrounds, are underrepresented in clinical trials. This poses significant challenges in understanding and quantifying the real-world value of new drugs in clinical practice including how market access of these new drugs may result in increasing existing health disparities. Other international organizations (ASCO, ACCC) have followed the same trend and emphasized how barriers can be broken down to increase inclusivity in clinical trial recruitment and participation. Valuable resources from other organizations (US Centers for Disease Control and Prevention) on setting principles for inclusive communication, such as using a healthy equity lens when framing and communicating information to specific patient groups by considering cultural and language barriers, would foster patient trial recruitment and study participation. Similarly, Duke Margolis Center for Health Policy’s recent publication emphasized how real-world data, “when sufficiently robust, has the propensity to fill knowledge gaps related to the long-term safety and real-world efficacy of treatments among and across patients that may be at a relatively higher risk of poor treatment outcomes due to distinct biological, environmental, discriminatory, or demographic factors.” However, as we previously discussed in another blog, the recently published guidance documents may miss the mark in fostering full diversity in clinical trials, covering other patient groups such as people with disabilities, those living in rural areas, or women from ethnic and racial minority groups.
Health equity is not exclusively a topic related to clinical trial development; attention also has been directed toward if and how integrating health inequities considerations in clinical and economic analyses of new products can be implemented during their market access assessments. Both NICE and CADTH placed equity and diversity as one of the key principles for their future strategic plans but do not formally integrate health inequities in their assessments. ICER has also produced a series of recommendations to improve considerations of health equity within every step of their health technology assessment (HTA) review; some of these specifically encourage the use of deliberative processes to highlight any existing disparities and assess the impact of new interventions and advise against the use of quantitative equity-informative economic evaluations. These are complex issues to handle both from a methodological (e.g., terminology standardization, data collection procedures, innovative economic modelling) and organizational perspective which would require both sponsors and decision-makers to increase collaboration and interaction across commonly diverse teams (epidemiologists, health outcomes and research community, implementation/pricing teams) addressing health equity considerations in different phases of product development.
In conclusion, as our society strives toward evidence-based healthcare, there is an urgent need for:
- All stakeholders: training on greater understanding of aspects of health inequity and its measurements
- All: wider use of real-world data and testing analytical methodologies to support health equity considerations
- Sponsors: close interaction of multiple, commonly separate teams to align early on evidence generation and synthesis, including the potential differential clinical benefit of health products for different patient groups
- Decision-makers: close collaboration on shared learnings and experiences on these issues including demonstration of case studies (see NICE example) to increase familiarity with the new challenges of operationalizing the integration of health inequities in formal assessments. This would also drive the publication of clear HTA guidance on this topic (minimum evidence quality requirements; acceptable data sources and analytical methodologies, such as data transferability, economic modelling; submission template changes) that will provide clarity and increase confidence on how the traditional HTA processes can shift by engaging to a dynamic data ecosystem centered on addressing health inequities.
Cross-country efforts such as the Joint Action Health Equity Europe (JAHEE) and global data repositories on health inequalities (such as WHO Health Inequality Data Repository) can guide international collaboration efforts and provide public platforms to facilitate this process.

Interested in learning more? Grammati Sarri will be at ISPOR Europe 2023! She will be moderating the panel discussion on “Gender and Health Equity in Health Care Decision-Making: How Women's Issues Continue to Be Neglected.” Click below to book a meeting with Cytel’s experts:
Read more from Perspectives on Enquiry and Evidence:
Sorry no results please clear the filters and try again
Can RWE Help Restore Decades of Health Inequalities? Yes, and Here’s How

FDA Increases Calls for Manufacturers to Ensure Trial Diversity, but Does It Fall Short of Addressing Health Inequalities in Product Development?

Writing a Successful Study Protocol for Real-World Evidence Studies


