Adaptive Multi-Arm Multi-Stage Designs: A Comparison of Methods

Written by Cyrus Mehta and Heather Struntz
The significant time and cost, as well as high failure rates, of clinical trials have necessitated innovative trial methodologies that are more efficient and informative. Adaptive clinical trial designs are a type of study design that allows pre-specified modifications to be made to the trial protocol based on interim looks at accumulating data. These designs are becoming increasingly popular as they offer several scientific, financial, strategic, and ethical advantages over traditional clinical trial designs, such as increased efficiency, reduced cost, and greater flexibility.
One such design type is multi-arm multi-stage designs, or MAMS designs. And the relatively recent cumulative MAMS design is, as we will explain below, the preferred option with respect to power.
What are multi-arm multi-stage (MAMS) designs?
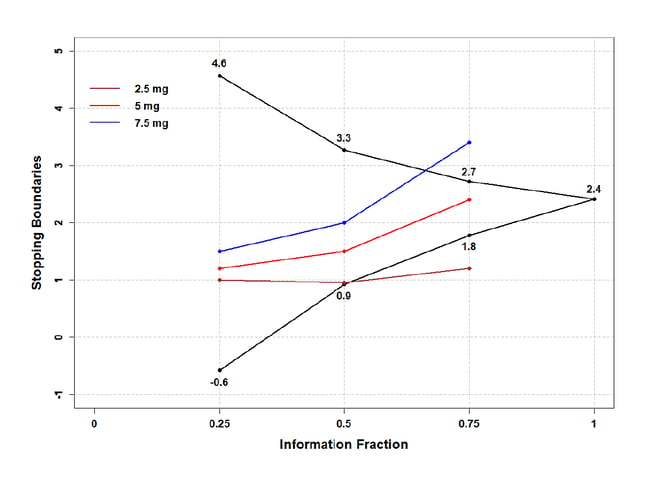
A multi-arm multi-stage (MAMS) design involves testing multiple treatment arms against a common control in a single trial in multiple stages, separated by unblinded looks at the data with the option to stop early for efficacy or futility, drop non-performing arms, or increase the sample size.
Two different approaches for MAMS designs can be distinguished:
-
- Stage-wise MAMS
- Cumulative MAMS
Both methods allow strong control of the family-wise error rate, that is, the probability of making at least one Type I error when testing multiple hypotheses. In stage-wise MAMS designs, this is achieved by converting the raw p-values observed at each stage into a single multiplicity-adjusted p-value and combining these stage-wise multiplicity-adjusted p-values into a single test statistic via the inverse normal combination function.
In the more recently developed cumulative MAMS design, a separate cumulative test statistic is constructed for each treatment vs. a control comparison, and efficacy can be claimed if at least one of these test statistics crosses a multiplicity-adjusted group sequential boundary.
How do stage-wise and cumulative MAMS designs compare with respect to power?
By comparing the stage‐wise MAMS and cumulative MAMS approaches under different scenarios and dose-selection rules (one, two, three, and four efficacious doses; Emax 1 and 2), we found that cumulative MAMS has greater power in all cases. For both methods and all scenarios, the power is smallest for the conservative drop-the-loser rules and increases with decreasing conservatism of these rules. Lastly, the power advantage of cumulative MAMS over stage-wise MAMS is greatest for conservative drop-the-loser rules and decreases with decreasing conservatism.
What are the potential benefits of MAMS designs?
MAMS designs offer several potential benefits in clinical trials, the primary of which is the ability to answer multiple research questions within the same trial. They are often more efficient, can lead to faster treatment discovery, improve resource allocation, maintain statistical rigor, provide more flexibility, and reduce the number of patients needed overall, all of which have the potential to provide patients with access to better therapies sooner.
The MAMS module in East®
The MAMS module in East® allows users to design, simulate, and monitor multi-arm multi-stage clinical trials, including stage-wise MAMS for normal, binomial, and time-to-event endpoints, for two-stage designs; and cumulative MAMS for normal and binomial endpoints, for any number of stages. The East module provides a wide variety of options to stop early, select treatment arms, and re-estimate the sample size, while strongly controlling the Type 1 error rate. It includes methods based on group sequential theory, as well as p-value combination approach.
Final takeaways
Adaptive multi-arm multi-stage clinical trial designs allow multiple therapies to be studied simultaneously, leading to a trial design that is more efficient and ethical. In a comparison between two approaches to MAMS — stage-wise and cumulative — cumulative MAMS designs are the preferred option, provided data are asymptotically normal and sample size is sufficiently large.
Interested in learning more about the comparison between stage-wise and cumulative MAMS designs? Click to download Cyrus's recent presentation, given at JSM 2023:
Read more from Perspectives on Enquiry and Evidence:
Sorry no results please clear the filters and try again

Cyrus Mehta on the Founding of Cytel

Adaptive Trials at the Mainstream of Drug Development

Embracing Innovation: Exploring the Design of Umbrella and Basket Trials


