Unravelling PICO: The Pillars of the European Joint Clinical Assessment

The European Union (EU) health technology assessment (HTA) regulation aims to improve the availability of innovative technologies for patients across the European Union (EU).1 It is also claimed to offer efficiency gains for manufacturers, due to one single EU-level submission vs. multiple parallel submissions to different HTA bodies.2 In this blog, we will first introduce the Joint Clinical Assessment (JCA), a legal requirement of the EU HTA regulation; the pillars that hold the JCA together, the PICO framework; and the consequential impact to manufacturers on reporting requirements based on multiple PICO.
What is the JCA and the PICO framework?
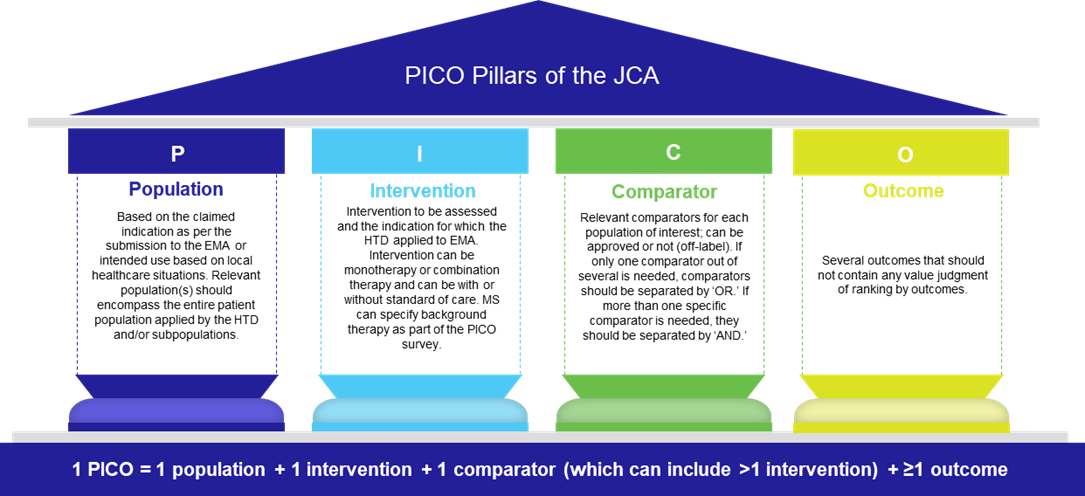
The EU HTA Regulation requires Member States to conduct JCA on the relative clinical effectiveness and safety of a new health technology. The overarching scope of the JCA is an all-encompassing assessment that addresses the specific needs requested by the Member State. The PICO framework provides a standard format for defining the assessment scope as population (P), intervention (I), comparator (C), and outcomes (O).
The scoping process for the JCA starts when the manufacturer submits a request for an assessment and ends when the final PICO is communicated to the manufacturer. Integral to this process (which is still under development) is the PICO survey, initiated by the Member State Coordination Group, in which the Member States are asked to describe PICO requirements needed for their national decision-making.
The Member States can also request additional information as part of the PICO survey, including details regarding potential treatment effect modifiers or background treatments. The assessor/co-assessor who develops the assessment scope then aims to consolidate the PICO(s) and presents these to the JCA Committee for Scientific Consistency and Quality, who in turn, validate the final PICOs.
What is the consequential impact to manufacturers on multiple PICO?
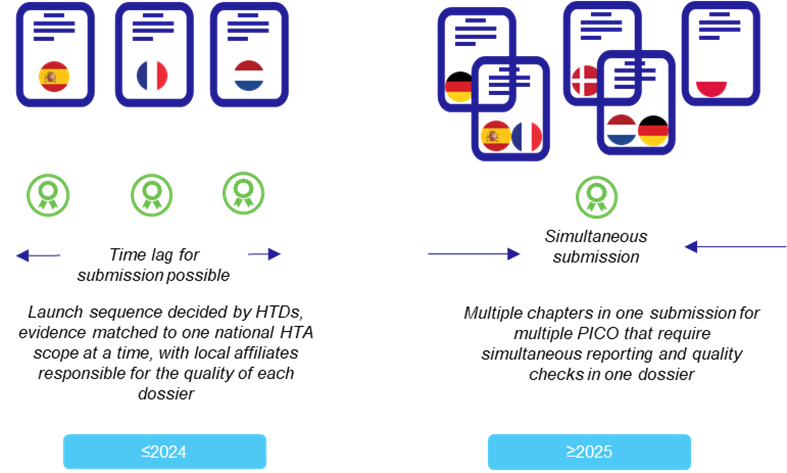
Although the practical guideline, “D4.2 Scoping Process,” provides an example of up to five PICOs consolidated,3 the reality is, for some indications, manufacturers may need to report on even more PICOs in one submission. Multiple PICO in one submission has consequences for data presentation in the JCA. For example, in first line non-small cell lung cancer, it was estimated that 10 population and comparator combinations would be relevant based on a hypothetical case study to the JCA, with a minimum of 28 different outcomes identified to cover multiple Member States.4 Now imagine that in one dossier!
The reporting needs by PICO across Member States have therefore inevitably expanded, and the quality control needed to complete reporting simultaneously at the European level cannot be ignored. In addition, considerations of PICO are not static at the time of initial data collection (i.e., prior to final validated PICOs). PICOs evolve, new evidence is constantly being published, and ultimately, manufacturers need to make decisions on which PICOs to submit to the JCA. Tools that can drill down by PICO and produce instant reporting may be necessary to ensure manufacturers can deliver timely, high-quality submission.

Interested in learning more? Join Maria Rizzo and co-presenters at ISPOR Europe in Copenhagen, to hear how technology and tools can help solve the PICO puzzle and navigate uncertainty in the JCA era. Click below to book a meeting with our experts:
References
- European Commission, “Regulation on Health Technology Assessment.”
- European Commission, “Questions and Answers: Adoption of Regulation on Health Technology Assessment,” 2021.
- EUnetHTA, “Practical Guideline: D4.2 Scoping Process,” v1.0, 2022.
- Van Engen, R. Kruger, J. Ryan, and P. Wagner, “HTA97 Impact of Additive PICOs in a European Joint Health Technology Assessment. A Hypothetical Case Study in Lung Cancer,” Value in Health 25 (12) (2022).
Read more from Perspectives on Enquiry and Evidence:
Sorry no results please clear the filters and try again
Mind the Health Gap: Is It Time to Reliably Measure the Impact of Health Inequity in Product Development and Assessment? Yes, It Is.

Reducing Independent Data Monitoring Committee Timelines: A Focus on Formal Interim Analyses

Writing a Successful Study Protocol for Real-World Evidence Studies


