On Kappler's 'Graphical Comparison of Simon two-stage designs'
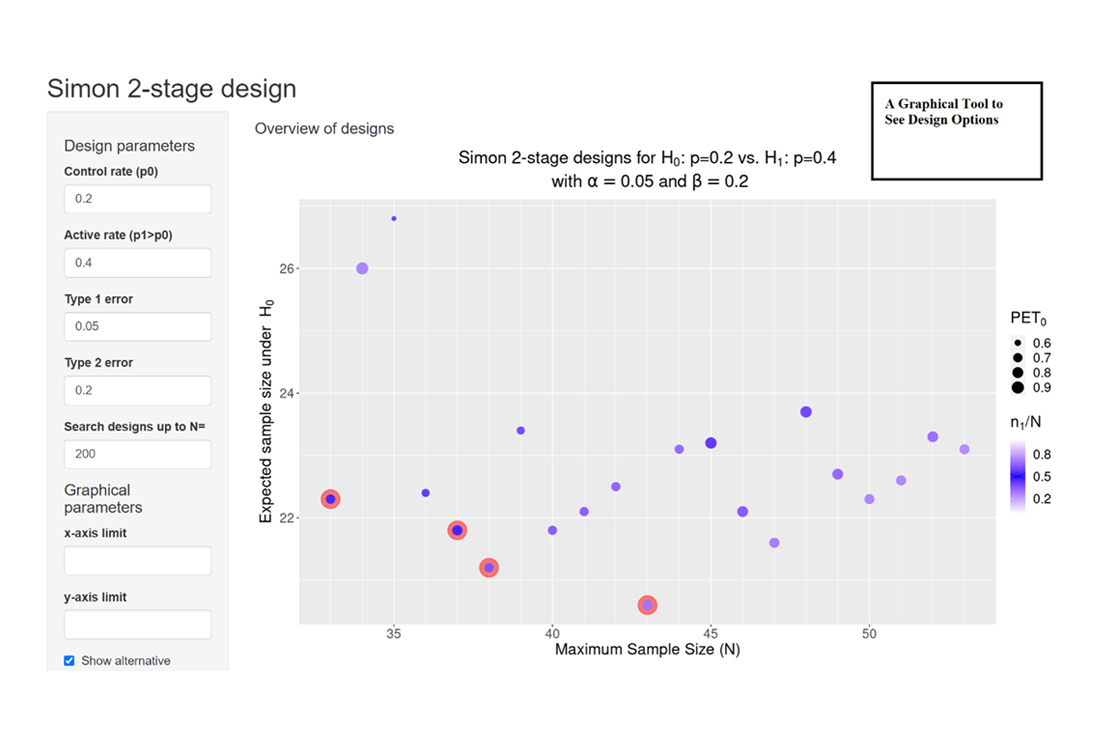
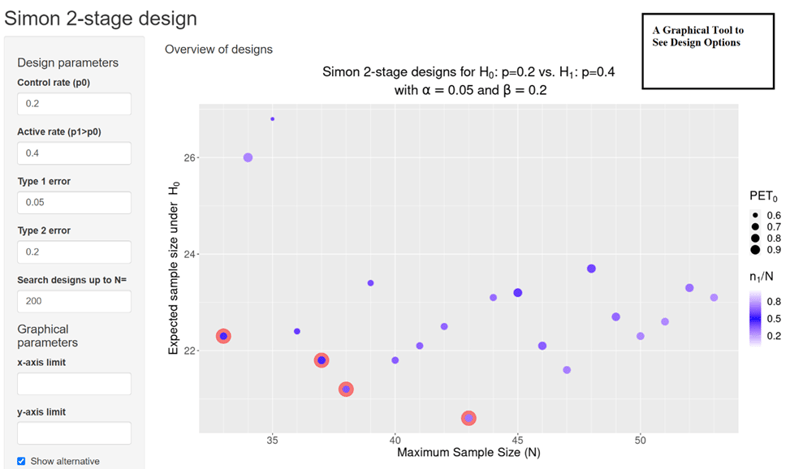
Clinical researchers, seeking to understand the statistical benefits of a common Phase 2 oncology design, now have a new visual aid thanks to Cytel's Martin Kappler. Phase 2 trials in oncology often rely on single-arm designs for a variety of clinical and ethical reasons. One of the most commonly used single-arm designs is Simon two-stage, which require sponsors to specify the maximum sample size. The variety of options available to sponsors, result in different operating characteristics such as average sample size, and the probability of stopping for futility after stage 1. These design characteristics are now easier for sponsors to assess and to compare.
What is a Simon two-stage design?
A design first proposed in 1989, Simon two-stage is still one of the most popular designs for Phase 2 oncology. A fairly simple and intuitive design, patients receive therapies in Stage 1. If a sufficient number of patients respond to said therapy, the trial continues through Stage 2. Otherwise it stops for futility.
How can sponsors use this graphical tool?
When sponsors choose a Simon two-stage design, they often decide to minimize the maximum sample size (Minimax design) or the expected sample size (Optimal design). Even so there are a variety of other two-stage designs, and it is important for sponsors to select those that meet their trial's needs. The new graphic enables sponsors to understand the operating characteristics of each of these designs, to facilitate a choice that is good for both clinical researchers and patients.
Where can sponsors see this graphical tool?
Martin will be presenting this new graphic at PSI 2022. Click below to see a full range of Cytel's keynotes and posters, and please come by our booth.
Abstract
Graphical comparison of Simon two-stage designs
Authors: Martin Kappler, Cytel Inc.
Simon’s two-stage design (Simon, 1989) is still one of the most often used designs in oncology phase 2 trials in single-arm trials testing a binary endpoint. After n1 patients (stage 1) the trial is continued to the final sample size N=n1+n2 only if the number of responders is above a fixed cutoff r. Otherwise, the trial is stopped for futility. The most popular choices for Simon two-stage designs are the minimax and the optimal design minimizing respectively the maximum and the expected sample size under the null hypothesis H0. Jung et al. (2004) proposed alternative admissible designs. But these are less known and rarely used as standard software packages don’t produce corresponding outputs.
The classical operating characteristics (OC) of a Simon two-stage design are its maximum and expected sample size. Other important OCs are the probability of early termination after stage 1 given H0 is true (PET0) and the ratio of stage 1 to the overall sample size (information fraction n1/N). However, these OC are often ignored, and the design decision is limited to the choice between Minimax and Optimal design.
An overview graphic was created and embedded in an R shiny web application to allow the visualization of the ensemble of all possible Simon two-stage designs together with the design OCs. The graphic provides a quick overview of admissible designs and their OCs facilitating clinical researchers the choice of the design.
References
Simon R. Optimal two-stage designs for phase II clinical trials. Contr Clin Trials, 1989, 10: 1-10
Jung SH, Lee T, Kim K, George SL. Admissible two-stage designs for phase II cancer clinical trials. Stat Med. 2004 Feb 28;23(4):561-9
About the Author of Blog:

Dr. Esha Senchaudhuri is a research and communications specialist, committed to helping scholars and scientists translate their research findings to public and private sector executives. At Cytel Esha leads content strategy and content production across the company's five business units. She received a doctorate from the London School of Economics in philosophy, and is a former early-career policy fellow of the American Academy of Arts and Sciences. She has taught medical ethics at the Harvard School of Public Health (TH Chan School), and sits on the Steering Committee of the Society for Women in Philosophy's Eastern Division, which is responsible for awarding the Distinguished Woman in Philosophy Award.


