Aligning Clinical Trial Design with Investment Priorities

Written by Natalia Muehlemann, Vice President, Clinical Development, and Ari Brettman, Senior Managing Director, Blackstone Life Sciences


When working with investors, it’s critical that drug and device developers consider how their clinical trial design aligns with investment priorities. Natalia Muehlemann and Ari Brettman discuss what investors look for when evaluating clinical development programs, and the value of simulation-guided design in informing decisions and facilitating communication with investors. Let’s take a closer look:
What do investors look for when evaluating clinical development programs?
When it comes to evaluating a clinical development program, it all starts with two things: 1) the strength of the scientific rationale of a product; and 2) how well it addresses an important unmet medical need.
Investors look to understand the validation of the therapeutic target if it’s a therapeutic product, or the engineering design if it’s a medtech product, to understand the nonclinical data that de-risks the human experience, which may or may not be limited at the time of a partnership opportunity. It involves a thorough review of the existing clinical data and often related regulatory interactions.
Pivotal trials may be in differing states of conception and design by the time investors engage with developers. So, it may also involve a review of not only the high-level design schema, but also the particulars around the operations of the study, the site plan, the enrollment prediction rates, and so on. And then, ultimately, what investors hope to get to in collaboration with their partners is a robust program — a clinical trial design that both parties believe in, that’s well-funded within the partnership structure that both feel is feasible, and that has a high likelihood of yielding not only an approval, but also an important medical innovation to patients.
What considerations are important when designing a clinical trial?
Investors are trying to optimize several parameters at once, and they often pivot around three axes: time, cost, and, most important, the probability of success of a funded study.
There are three options we can consider: an optimistic scenario, a conservative scenario, and an adaptive approach.
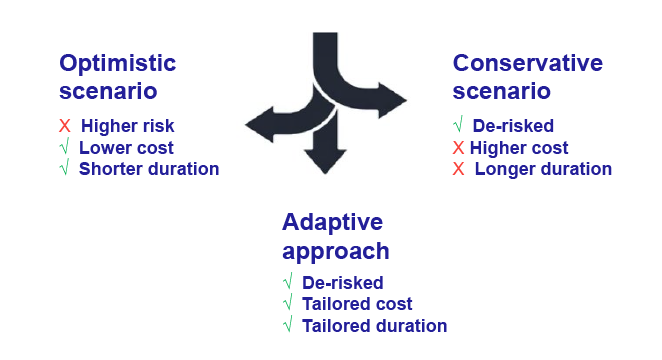
An optimistic design puts a lot of burden on the product to perform above expectations, which has a higher risk associated with it, but often confirms benefits in terms of time and cost. A conservative scenario often maps what might be regarded as the minimum viable product profile of a pharmaceutical product or device, where we are very comfortable that the primary endpoint can be met but is very costly and takes more time than anyone wants.
The best of both worlds is an adaptive approach, in which there are multiple parameters that can be adapted during the course of the study, taking into account data as it’s being collected. But this often requires a lot of expertise both in terms of clinical experience and quantitative modeling and simulation.
Cytel’s approach to simulation-guided trial design
Contrary to a fixed design that does not have any flexibility, adaptive designs allow sponsors to stop earlier for futility or efficacy, increase sample size, or enrich the population. There is no one-size-fits-all design because the choice of different scenarios and different designs really depends on your clinical, regulatory, operational, and financial considerations. Cytel’s award-winning simulation platform Solara® helps explore different combinations and permutations of parameters, resulting in about 50,000 trial design models. The platform also allows sponsors to select optimal designs that meet the needs of the investor and the company. The figure below provides a simple visualization of trade-offs for decision-making between power, duration, costs, robustness score, and time to interim analysis (decision). Robustness score is an important parameter that reflects the probability of the study’s success over the range of assumptions. Time to interim analysis and cumulative probability of stopping for efficacy or futility at interim analysis are also key to design selection.
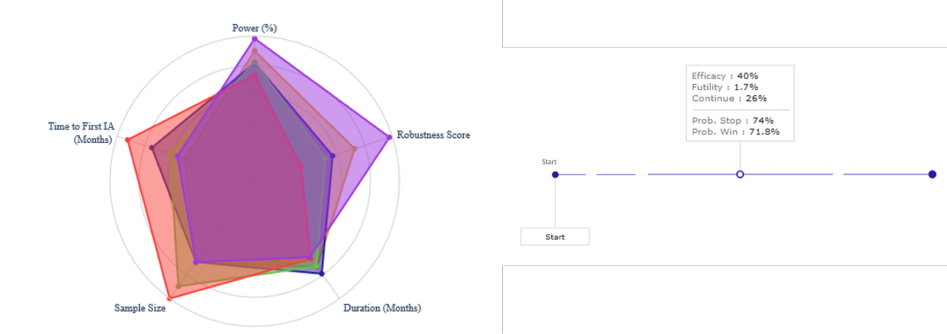
When exploring all possible options, how do investors balance priorities?
From the investor perspective, the initial priority is to optimize the robustness of a study as much as possible in order to minimize failing for avoidable design decisions and end up with a study that’s resilient to variance and any number of assumptions. The most common uncertainty is the expected effect size; there could be minimal clinically meaningful effect or a range bound based on prior data. There is also always a variability in enrollment rates. Working with Cytel, we can define the reasonable range of those estimates, and then once we get comfortable that we’re in the target zone, layering on time and cost assumptions to narrow it down and find where there is room for compromise.
Investors are often very focused on the operating characteristics of making decisions on the trial at different time points, when a certain amount of the budget has gone in, because when something is looking like it’s going to fail, knowing that as soon as possible and saving the capital for another opportunity is in everyone’s best interest.
Of course, time is always of the essence, for both investors and developers, and being able to explore a broad range of designs very rapidly, where you can see where the global and local optima are very quickly, and very rapidly prioritize resources toward that, is only possible with powerful simulation techniques.
How can R&D teams communicate their clinical plans to investors more efficiently?
This relates not only to investors, but to boards and even the financial planning functions within a business. On the business side and on the investment side, it often comes down to discussion around an operating plan, knowing how much to budget, and when the next budgeting decisions are going to be made — when we will get a read on whether we’re stopping or continuing. And simulation allows a pretty easy cross-functional way of communicating those decisions and creating a common language for how we integrate all the prior data, both from previous trials and from the same trial.
Scenario planning is very time-consuming within boards and business units, so having a quantitative language that can be shared across all the functions within a project is very critical.
For more information, watch our webinar “Balancing Priorities in Therapeutics Development: Simulation-Guided Decision-Making”:
 About Natalia Muehlemann
About Natalia Muehlemann
Natalia Muehlemann is Vice President, Therapeutics Development Team, at Cytel. Natalia Muhlemann, MD, MBA, has over 20 years of experience in general management, clinical development, and business development in the life sciences. Dr. Muhlemann joined Cytel in 2020, and prior to Cytel, served as Global Category Head, Acute Care - Oncology - Devices at Nestle Health Sciences. She acts as an Expert Jury member for the European Commission’s Innovation Council. Dr. Muhlemann holds an MD and an MBA (IMD) and professional certifications in statistics and data science.
 About Ari Brettman
About Ari Brettman
Ari Brettman is a Senior Managing Director at Blackstone Life Sciences, focusing on investments across the firm’s portfolio. Dr. Brettman received his M.D. from Duke University and his AB in History and Science from Harvard College before completing his residency in internal medicine and fellowship in cardiology at Massachusetts General Hospital. He is a founding member of the Board of Directors of Anthos Therapeutics and AvenCell and has served on the boards of Praxis Precision Medicines and Essa Pharmaceuticals.
Read more from Perspectives on Enquiry and Evidence:
Sorry no results please clear the filters and try again
How to Ensure Your Adaptive Trial Is Appropriate for Regulatory Submission

How to Ask the Right Questions at an Authority Meeting

Writing a Successful Study Protocol for Real-World Evidence Studies


