Society of Clinical Trials names TOGETHER "Trial of the Year"

Early in the pandemic, it became clear that many of the COVID-19 therapies being tested in wealthier nations, were not taking into consideration the accessibility of these medicines in low- and middle-income countries (LMICs). An adaptive platform trial was quickly developed to test repurposed medicines in LMICs to ensure affordable and equitable access. TOGETHER has now launched in Brazil, the Democratic Republic of Congo, Pakistan, South Africa and Vietnam. It has enrolled over 6000 patients and just received the Society of Clinical Trial’s David Sackett Trial of the Year Award for 2021.
TOGETHER Trial
Led by Principal Researcher Dr. Edward Mills (Cytel & McMaster), TOGETHER Trial studied the effects of therapies on hospitalization rates for patients diagnosed with COVID-19 who also have a high-risk due to age or co-morbidities.
At the time of launch, Dr. Mills had explained that reducing hospitalization was “a vital approach for areas around the globe that simply cannot cope with greater hospital demand." While hospitals across the world were struggling to respond to the high rates of hospitalization, this issue had particular significance in LMICs.
Ed had added, “[I]mplementing such a trial is no easy task. A much deeper level of statistical insight needs to feed into design and analysis stages, and operational complexities are an order of magnitude greater.”
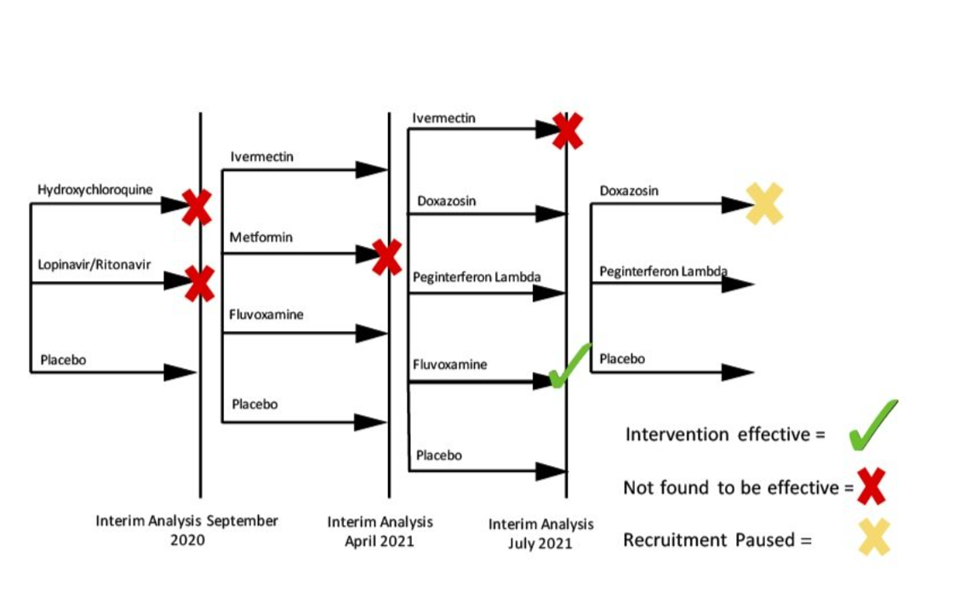
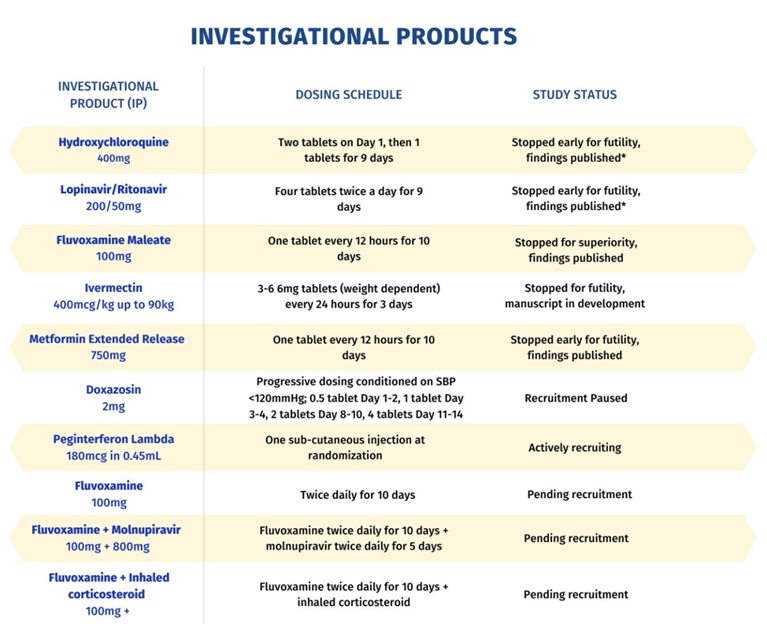
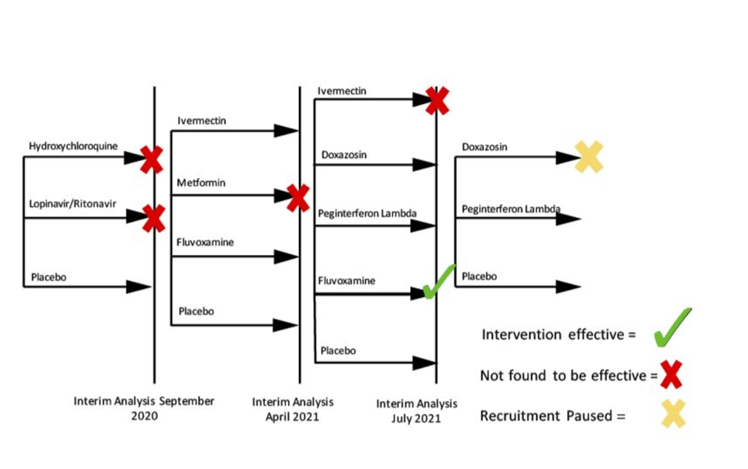
The trial launched in July 2020, with Hydroxychloroquine and Lopinavir/Ritonavir tested against a placebo. Both therapies were dropped during an initial interim analysis in September 2020. Seven additional therapies have been added.
Why an adaptive platform design?
"Platform trials are not only efficient at identifying therapies that work, they tend to eliminate poorly performing therapies, making it a double win.” – Kyle Wathen, VP of Scientific Strategy and Innovation (Designer of I-SPY2).
TOGETHER Trial was designed as an adaptive platform trial, a design with the flexibility to add and drop medicines as data becomes available in real-time. Amongst the benefits of an adaptive platform trial is that multiple therapies share a comparator arm, thereby reducing the need for patients to enroll into placebo. Sharing a study protocol also makes it easier to compare the effectiveness of each of the therapies to each other.
So far, TOGETHER has been able to evaluate 11 interventions within a two year period with the earliest results arriving just months after the trial launched. Within the first year, TOGETHER had found rigorous evidence that led to four therapies being eliminated from consideration. It also discovered the effectiveness of Fluvoxamine for reducing hospital visits for those with comorbidities.
The David Sackett Award
David Sackett was widely regarded as the Father of Evidence Based Medicine. He noted that clinicians often had trouble keeping up to date with research in the field, as new evidence was outpacing the ability of clinicians to digest it. According to early analysis, a doctor would have to reach 17 articles a day just to keep up with methodological research. Sackett and his colleagues mobilized new ways to make groundbreaking research more easily digestible, leaving a lasting impact on areas like comparative effectiveness research.
The Society for Clinical Trials uses the David Sackett Award to recognize clinical trials that accomplish five fundamental goals:
- Improve the lot of humanity;
- Offer substantial, beneficial improvement in healthcare;
- Reflect expertise in both subject-matter and trial design while demonstrating concern for patients;
- Overcome obstacles in implementation;
- Present the design, execution and results of the trial in a way that is a model of clarity and intellectual soundness.
About the Author of Blog:
Dr. Esha Senchaudhuri is a research and communications specialist, committed to helping scholars and scientists translate their research findings to public and private sector executives. At Cytel Esha leads content strategy and content production across the company's five business units. She received a doctorate from the London School of Economics in philosophy, and is a former early-career policy fellow of the American Academy of Arts and Sciences. She has taught medical ethics at the Harvard School of Public Health (TH Chan School), and sits on the Steering Committee of the Society for Women in Philosophy's Eastern Division, which is responsible for awarding the Distinguished Woman in Philosophy Award.


