Platform Trials, Master Protocols, and Challenges in Execution
How can we build an efficient statistical protocol for a clinical trial, if we do not know the therapies that will be tested nor the populations on whom the testing will occur? This is the challenge confronting those working to design an ever-increasing number of platform trials. In a recent webinar, Dr. Kyle Wathen, Cytel's Vice President of Scientific Strategy and Innovation, discussed a method using platform trials to show how to build such trials.
Platform Trials – A brief overview
Trials like I-SPY2, Janssen’s trials on autism spectrum disorder, and a number of COVID therapies, the set of trial designs that fall under the purview of master protocols is growing in number as well as nuance. Basket trials, umbrella trials, and platform trials not only raise design challenges, they all require enhanced operational know-how to adequately measure treatment effect. Still, their value to expediting drug development is unparalleled.
A master protocol can either test a single investigational drug or drug combination in many populations (a basket trial), or many investigational drugs or drug combinations in a single population (an umbrella trial). A platform trial can combine both of these elements to test several investigational therapies in heterogeneous populations where new therapies are added over time via an Intervention Specific Appendix (ISA).
The benefits of a platform trial are that both research infrastructure and data collected can be shared among appropriate investigational therapies. This decreases cost by spreading and scaling resource use, while also providing for more robust analyses. The challenges lie in many areas including statistics, operations, and trial execution, all of which can impact the performance of the design.
A further challenge, examined in a recent webinar by Cytel's Dr. Kyle Wathen, is the difficulty of building a protocol, when it is unknown which therapies and populations will eventually be tested using it.
Using Intervention Specific Appendices (ISA) for information sharing
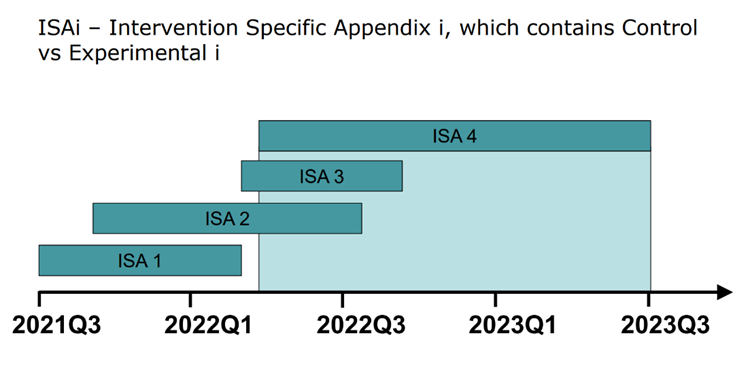
Each investigational drug added to a platform trial will have its own ISA, which contains information on both comparator and treatment. As the trials progress, a diagram such as the one above can demonstrate which interventions were being tested and when. This not only provides a simple model for what is being tested, it quickly becomes clear that information sharing between investigational drugs is easiest when ISAs overlaps. When they don’t overlap, careful consideration must be taken in sharing information between non-concurrent ISAs.
A number of quantitative strategies can be used to adjust for borrowing patients across ISAs. It is possible to discount patients enrolled before an ISA began, for example, or create a model for time trends. Hierarchical borrowing is also a possibility, although each of these advanced approaches requires significant statistical expertise.
Building the right randomization strategy for a platform trial
Randomization within the context of a platform trial can either be a one- or a two-step strategy. The traditional mode of randomization (the one-step strategy) requires greater screening when directed at a master protocol trial. The two-step strategy for ISAs can raise challenges about informed consent.
In a one-step strategy, a patient is directly randomized to a treatment. Alternatively, patients can first be randomized to an ISA and then to a treatment within the ISA. The two-step solution creates additional flexibility in that either a patient or a site, might be opposed to one or more of the ISAs on a platform. One must carefully consider the trade-offs of either a one-step or two-step approach.
Considerations about statistical analysis plans when implementing platform trials
Building SAPs for platform trials raise specific challenges. Statisticians need to be familiar with methods that account for population heterogeneity, and rules for stopping for efficacy and futility have added considerations when compared to more traditional approaches.
Dr. Wathen warns that developing a master protocol approach requires additional considerations and an extensive simulation study in order to fully understand the performance of various options. He also discussed the importance of carefully considering future interventions or subgroups that may be added in order to help avoid potential issues when they are added.
Trial design optimization with platform trials
According to Dr. Wathen, designing an efficient platform trial is very similar to designing a number of more traditional trials at once. There is an added feature, of course, that ISAs executed concurrently make data-sharing easier. This means that decision-rules for a given platform likely look quite different than traditional trials and must be thoroughly explored by simulation in the design process to account for this. OCTOPUS (https://kwathen.github.io/OCTOPUS/) , an R-package he developed while at Janssen, can help simulate different platform designs.
To view the full webinar, click below.
About the Author of Blog:

Dr. Esha Senchaudhuri is a research and communications specialist, committed to helping scholars and scientists translate their research findings to public and private sector executives. At Cytel Esha leads content strategy and content production across the company's five business units. She received a doctorate from the London School of Economics in philosophy, and is a former early-career policy fellow of the American Academy of Arts and Sciences. She has taught medical ethics at the Harvard School of Public Health (TH Chan School), and sits on the Steering Committee of the Society for Women in Philosophy's Eastern Division, which is responsible for awarding the Distinguished Woman in Philosophy Award.


