Living SLRs and the Rise of Digital Technology

Written by Marie Diamond and Maria Rizzo
Systematic literature reviews (SLR) are essential to informing healthcare decision-making and are pivotal for reimbursement submissions to health technology assessment (HTA) bodies. One limitation of SLRs, however, is that they quickly become outdated due to the continuous publication of new literature. The living systematic review (LSR) model can overcome this challenge by incorporating relevant new evidence as it is published.
Living approaches to evidence curation have been gaining interest where updated, living evidence can have a meaningful impact on health inequalities and/or in clinical guidelines to help speed patient access to technologies. Although there is a lack of clear guidance and standards for LSRs, we can draw upon the literature to learn how to successfully deliver LSRs (see Figure 1).1
Figure 1: Recommended LSR process
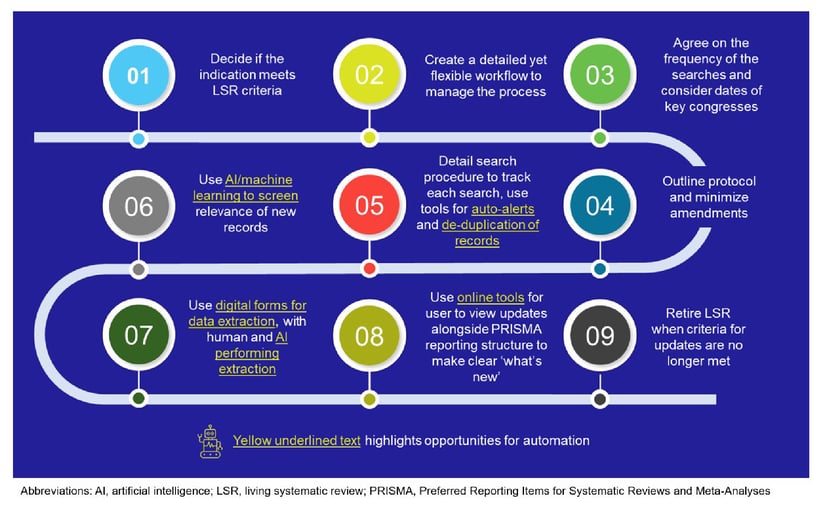
What is an LSR?
LSRs have the same fundamentals and steps as standard SLRs, but they use a process that facilitates continuous monitoring of the literature, incorporating relevant data as it becomes available. Due to the complexity of LSRs and the processing speed required to maintain them, researchers often employ digital technologies including artificial intelligence (AI) or machine learning (ML) tools; opportunities to use automated tools are available at just about every stage of the LSR.1,2
What are the opportunities for digital technologies in LSRs?
The use of some form of digital technology in an LSR is needed to be able to manage the pace of updates. Examples of digital tools used in SLRs include ones that can assist with running a search update and deduplicating the records (e.g., LiveSTART, EPPI-Reviewer). AI tools can help screen for relevant records (e.g., LiveSTART, Distiller SR, EPPI-Reviewer, SWIFT-Active Screener)2,3,4 and extract data (e.g., LiveRef auto extractor, PICO Portal, SWIFT-Active Screener).2,5 In addition, reporting platforms are available that can automatically synthesize the data and produce living Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagrams and reports (e.g., LiveSLR®, RobotReviewer, PICO Portal)2,6,7 Studies have shown that the use of ML tools can generate a workload savings of between 14% and 95%.8 The rise in digital tools is reflected in the PRISMA 2020 checklist, which requires authors to report the use of automation in SLRs.9
Which organizations are advocating for LSR methods and the use of digital technology to enable the process?
Cochrane has been a key organization that has utilized a living model on some of its systematic reviews and has published LSR methods and guidance on its website and in peer-reviewed journals.10 It recommends considering an LSR for healthcare topics that are rapidly changing, where new evidence will likely impact decision-making.11 Cochrane guidance also describes the use of “tech enablers” to maximize efficiencies required in a LSR using text mining and ML (e.g., randomized clinical trial [RCT] classifiers).
The Australian Living Evidence Collaboration is another group at the forefront of developing LSR methods and sharing its experiences with the process.12 Regarding HTAs, the European Commission’s Next Generation Health Technology Assessment (HTx) project is creating a framework for real-time evidence curation to support assessment of health technologies throughout Europe.13 Lastly, a core strategic pillar for the National Institute for Health and Care Excellence (NICE) is to produce dynamic, living guideline recommendations, presented in an interactive format.14 Although these organizations have not yet issued a statement on use of AI or ML in LSRs, NICE in its strategic priorities has focused on the need for an interactive, digitalized format to host living guidelines.
Why is Cytel well positioned to adapt to LSRs?
In addition to the many years of experience that Cytel has with producing SLRs to support HTA submissions, we have developed LiveSLR®, an online, digital platform to host up-to-date evidence from SLRs, with automated reporting that allows customization of the SLR evidence base via “PICO” (i.e., population, intervention, comparators, and outcomes) filters. Cytel has also developed tools, including LiveSTART and LiveRef auto-extractor to enable screening, and data extraction to recognize the efficiencies of AI-assisted reviews. The ability to turn “on or off” AI-assisted reviews is empowered by LiveSTREAM, which is a literature review platform tailored to Cytel’s AI and automation solutions, LiveSTART and LiveSLR®.
Why should the life science industry adopt LSRs?
Preparing a global dossier that addresses multiple regional and local decision problems requires the use of technology and tools. The new European HTA Regulation, the Canadian Agency for Drugs and Technologies in Health dossier procedures, and the United States Inflation Reduction Act all require a wide variety of data across multiple population, comparators, and geography, while staying up to date as new evidence is published. Using LSR methods and technologies such as LiveSLR® allows industry to capture the breadth of data, monitor new evidence as it is published, and make informed decisions on when to execute a full human update. This ensures industry can meet the demands of HTAs in an ever-depleting timeline from regulatory licensing to reimbursement applications.
|
Interested in learning more? Cytel’s Real-World and Advanced Analytics team will be at ISPOR US Booth #1018. Click to book a LiveSLR® demo with our experts: |

About Marie Diamond
Marie Diamond is Information Specialist and Senior Research Consultant at Cytel HEOR & RWE. She is bibliographic database researcher with broad experience in literature research and screening.
 About Maria Rizzo
About Maria Rizzo
Maria Rizzo is Vice President, Evidence Reviews, Software, and AI Solutions, at Cytel HEOR. She has 16 years of experience in evidence synthesis from payer application of literature reviews to life science consultancy. Maria specializes in the use of tools to enhance literature reviews and conducts network meta-analyses to gather evidence to support pharmaceutical companies in gaining market access.
Notes
1 Diamond, M., Valbuena-Fajardo, J.A., Appiah, K., & Rizzo, M. (2023). Examining guidance and key principles for conducting living systematic reviews: A methods review. ISPOR EU, November 12–15, Copenhagen, Denmark.
2 Schmidt, L., Sinyor, M., Webb, R.T., Marshall, C., Knipe, D., Eyles, E.C., John, A., Gunnell, D., & Higgins, J.P.T. (2023). A narrative review of recent tools and innovations toward automating living systematic reviews and evidence syntheses. ZEFQ, 181.
3 Liu, J., Jafar, R., Girard, L.A., Thorlund, K., Rizzo, M., & Forsythe, A. (2023). Improving efficiency of Living Systematic Literature Reviews (SLR) with Artificial Intelligence (AI)-assisted extraction of Population, Intervention/Comparator, Outcome, and Study design (P-I/C-O-S). ISPOR EU, November 12–15, Copenhagen, Denmark.
4 Liu, J., Jafar, R., Girard, L.A., Thorlund, K., & Forsythe, A. (2023). Re-training of the artificial intelligence (AI) tool LIVESTART with additional datasets: Updated accuracy in the title and abstract review stage of systematic literature reviews (SLR). Value in Health, 26(S6).
5 Liu, J., Jafar, R., Girard, L.A., Thorlund, K., & Forsythe, A. (2023). Update on the LIVESTART artificial intelligence (AI) systematic literature review (SLR) tool – Can it aid in limited data extraction for LIVEREF. Value in Health, 26(S6).
6 Liu, R., Agranat, J., Rizzo, M., & Forsythe, A. Exploring efficiency of living systematic literature review (SLR) tool for submissions of clinical evidence to National Institute for Health and Care Excellence (NICE) by combining interventional and real-world evidence (RWE). Value in Health, 26(s12).
7 Liu, R., Buer, A., Rizzo, M., Sarri, G., & Forsythe, A. The data lifecycle: Towards a standardized relational data model to support living systematic literature reviews? – Use case demonstration with LiveSLR®. Value in Health, 26(S6).
8 Chappell, M., Edwards, M., Watkins, D., Marshall, C., & Graziadio, S. (2023). Machine learning for accelerating screening in evidence reviews. Cochrane Evidence Synthesis and Methods, 1(5).
9 Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P., & Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372(71).
10 Cochrane Community. Living systematic reviews.
11 Butler, A.R., Hartmann-Boyce, J., Livingstone-Banks, J., Turner, T., & Lindson, N. (2024). Optimizing process and methods for a living systematic review: 30 search updates and three review updates later. Journal of Clinical Epidemiology, 166.
12 Australian Living Evidence Collaboration.
13 Next Generation Health Technology Assessment (HTx Project).
14 National Institute for Health and Care Excellence (NICE). (2021). NICE strategy 2021 to 2026: Dynamic, collaborative, excellent.
Read more from Cytel's Perspectives:
Sorry no results please clear the filters and try again

Early Planning Strategies for External Control Arms in HTA and Regulatory Submissions
The New EU HTA Landscape: Insights on Indirect Evidence

Key Elements and Implications of the Draft EU JCA Implementing Act


