Embracing AI and ML in Medical Devices: FDA’s Total Product Lifecycle-Based Regulatory Framework

Written by Fei Tang, RWE Senior Research Consultant, and Paul Arora, Assistant Professor (Status), Dalla Lana School of Public Health, University of Toronto
The Food and Drug Administration (FDA) has long been committed to innovative approaches to the regulation of medical device software and other digital health technologies. One such innovation is the use of artificial intelligence (AI) and machine learning (ML) in software, which has the potential to learn from real-world use and experience and improve its performance. The FDA’s vision is that AI/ML-based Software as a Medical Device (SaMD) will deliver safe and effective software functionality that improves the quality of care that patients receive.
In 2019, the FDA published a Proposed Regulatory Framework for Modifications to AI/ML-based SaMD. This framework would allow the FDA and manufacturers to evaluate and monitor a software product from its premarket development through postmarket performance. The framework embraces the iterative improvement power of AI/ML-based SaMD, while ensuring the safety and effectiveness of the software.
The framework includes a “Predetermined Change Control Plan” in premarket submissions. This plan would include the types of anticipated modifications — referred to as the “SaMD Pre-Specifications” — and the associated methodology being used to implement those changes in a controlled manner that manages risks to patients. The FDA expressed an expectation for transparency and real-world performance monitoring by manufacturers.
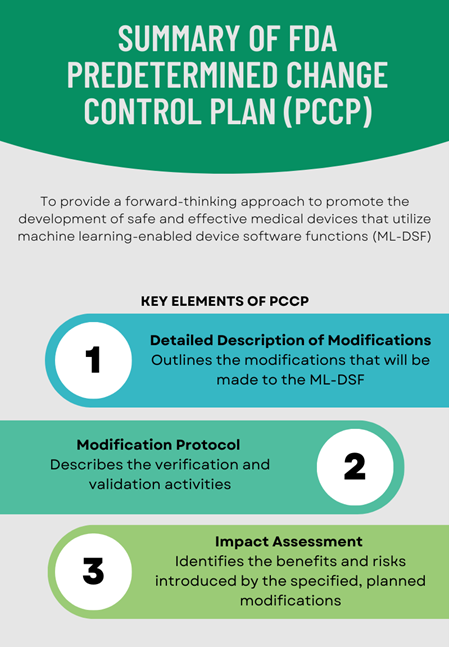
Stakeholders have provided many suggestions for further development of the proposed regulatory framework for AI/ML-based SaMD, including for the Predetermined Change Control Plan. The FDA continued to update the proposed framework for AI/ML-based SaMD, including publishing a Marketing Submission Recommendation for a Predetermined Change Control Plan (PCCP) for AI/ML-Enabled Device Software Functions (ML-DSF) in April 2023. Figure 1 presents a summary of the essential components that the FDA recommends to be included in the PCCP.
Figure 1: Summary of the Key Elements of FDA’s Predetermined Change Control Plan Published in April 2023
The FDA is also supporting numerous regulatory science research efforts to develop methods to evaluate AI/ML-based medical software. These efforts are being conducted through collaborations with leading researchers at the Centers for Excellence in Regulatory Science and Innovation (CERSIs) at the University of California San Francisco (UCSF), Stanford University, and Johns Hopkins University.
However, adopting AI and ML in medical devices is not without challenges. Stakeholders have described the need for clarity on Real-World Performance (RWP) monitoring for AI/ML software. The FDA plans to work with stakeholders piloting the RWP process for AI/ML-based SaMD.
The FDA’s total product lifecycle-based regulatory framework promises to harness the power of AI and ML for medical devices, while ensuring safety and effectiveness. The challenges lie in the implementation details, but with continued stakeholder engagement and regulatory science research, the promise of AI and ML in medical devices can be realized.
As a leading consulting company, Cytel is well-positioned to assist device manufacturers in navigating the Real-World Evidence (RWE) component of the FDA’s guidance for ML in medical devices. With our deep expertise in data science and biostatistics, we can help manufacturers design and implement robust RWE strategies that align with FDA expectations. Our team can guide you in collecting, analyzing, and interpreting real-world data to demonstrate the safety and effectiveness of your AI/ML-based SaMD. By partnering with Cytel, manufacturers can confidently navigate the complexities of the FDA’s total product lifecycle-based regulatory framework and bring innovative, safe, and effective devices to market.
Have questions? Contact us!
Read more from Perspectives on Enquiry & Evidence:
Sorry no results please clear the filters and try again

New FDA Guidelines on Pediatric Studies and Potential Effects on Opportunities for Market Exclusivity

FDA Increases Calls for Manufacturers to Ensure Trial Diversity, but Does It Fall Short of Addressing Health Inequalities in Product Development?



