
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

March 11, 2024
In 2023, rare diseases accounted for 30% of product pipeline under development, about half of which comprising...
Read article

January 15, 2024
Navigating the Clinical Development Landscape: Insights for Success in 2024
After explosive and frenetic activity in the clinical trial industry during the COVID era, the past two years have seen...
Read article

October 30, 2023
Managing Uncertainty: Simulation-Based Assurance in Clinical Trial Design
The past two decades have seen the adoption of great innovation in clinical trial design. Statisticians have risen to...
Read article

August 4, 2023
Bayesian Methods for Strategic Clinical Trial Design
“There is always the risk that interim analyses might occur after the Sufficient Information Threshold has been...
Read article

August 1, 2023
Dynamic Bayesian Borrowing to Bolster Limited Sample Sizes in Rare Indications
Evaluating the efficacy and safety of novel therapies in rare indications can be challenging due to the difficulty of...
Read article
January 25, 2023
Bayesian Approach in Oncology Trials
People think in Bayesian terms all the time: we use prior information and the evidence at hand to make decisions in our...
Read article

January 17, 2023
Bayesian Strategies in Rare Diseases
When it comes to rare diseases, a handful of major challenges to drug development arise. Bayesians strategies have...
Read article

January 13, 2023
Bayesian Methods across the Clinical Development Journey
Bayesian methods, with their ability to facilitate flexibility and learning, are often associated with early-phase...
Read article

January 12, 2023
Why Are There Not More Bayesian Clinical Trials?
Statistical methods have long been fundamental to drug development, and advancements in the last few decades in...
Read article

January 4, 2023
A Look Ahead for 2023
Returning to Cytel after the winter holidays, I am excited to begin a year that will likely prove memorable for both my...
Read article

December 29, 2022
Top Perspectives Articles of 2022
Perspectives on Enquiry and Evidence explores a wide variety of topics within clinical trial design and data science in...
Read article

December 27, 2022
Top Bayesian Topics of 2022
Bayesian methods have been playing a key role in transforming clinical research, providing a variety of new...
Read article

December 21, 2022
Top Interviews of 2022: Industry Voices and Career Perspectives
Perspectives on Enquiry and Evidence features two recurring interview series: Our new Industry Voices series, in which...
Read article

December 16, 2022
Topics in Bayesian Statistical Methods
Bayesian methods have been playing a key role in transforming clinical research, and Bayesian topics are frequently...
Read article

November 29, 2022
Bayesian Adaptive Clinical Trial Designs: INLA vs. MCMC
Bayesian methods have continuously played a key role in transforming clinical research in therapeutic areas such as...
Read article

November 18, 2022
Industry Voices: Dr. Parvin Fardipour on New Horizons in Data Science
In the following interview, Dr. Parvin Fardipour, Quantitative Strategies & Data Science, sits down with Heather...
Read article

October 5, 2022
Platform Trials, Can they Benefit Animal Studies?
Master protocols and platform clinical trials have become an innovative and efficient approach to testing multiple...
Read article

October 4, 2022
MCMC vs. INLA in Bayesian Adaptive Clinical Trial Designs
Integrated Nested Laplacian Approximations (or INLA) are now starting to be used by statisticians as a key tool for...
Read article

October 3, 2022
Bayesian Methods for Historical Borrowing: Conjugate Priors
The wider availability of electronic health data, medical registries, and even larger proprietary datasets means that...
Read article

September 12, 2022
On Frequentist and Bayesian Sequential Clinical Trial Designs
In clinical trials, patient enrollment is often staggered, with data collected sequentially. When designing a clinical...
Read article

September 9, 2022
Developing Synthetic Control Arms Using Bayesian Models
A new trend has emerged over the last decade that has changed the way many clinical trials are conducted. Unlike...
Read article

September 2, 2022
Summer Weekend Read Roundup
Last week, we featured our final Summer Weekend Read, the last in a series designed to showcase some of our most recent...
Read article


August 3, 2022
The Uses of Bayesian Methods in Late-Phase Clinical Trial Strategy
A number of late-phase clinical trial sponsors remain hesitant to employ Bayesian approaches in confirmatory settings,...
Read article

June 22, 2022
How to Determine if Your Clinical Trial Has Sufficient Data?
It can be difficult to estimate just how much time and data you need to address the multitude of considerations that...
Read article

May 24, 2022
Leveraging Advanced Statistical Software to Optimize Clinical Development
Traditionally, clinical trials are expensive, long in duration, and have low success rate. But with the advent of rich...
Read article

April 21, 2022
Bayesian Statistics and Its Applications: New Webinar by Professor Yuan Ji
Sophisticated Bayesian Methods are gaining a lot of traction as they bring flexibility and speed to clinical trial...
Read article

April 12, 2022
Career Perspectives: Interview with Charles Warne, Associate Director of Biostatistics
In this edition of the Career Perspectives series, I interview Charles Warne, Associate Director of Biostatistics at...
Read article

April 1, 2022
A Spotlight on Our Scientific Community
At the end of every year, scientists from across Cytel’s business units are nominated for Cytel’s Spotlight Awards,...
Read article

March 11, 2022
WINTER WEEKEND READ: Increased Adoption of Innovative Designs
Every year, sponsors hesitating to use a complex innovative clinical trial design routinely miss opportunities to...
Read article

January 19, 2022
What’s Ahead for Clinical Trial Design?
The past two years have been transformative for Cytel. Most notably, the global COVID-19 pandemic unleashed an...
Read article

October 6, 2021
The Rapidly Evolving Need for Master Protocols
Master Protocols are advanced and innovative clinical trial designs that can evaluate multiple therapies and disease...
Read article

October 1, 2021
Dr. Kyle Wathen talks about the Need for Statistical Innovation
The COVID-19 pandemic elevated the challenge of designing and executing clinical trials within a substantially...
Read article

September 24, 2021
ICT Magazine Interviews Dr. Yannis Jemiai on the Benefits of Bayesian
We may be familiar with adaptive designs, but their complexity has made them difficult to implement and their benefits...
Read article

September 15, 2021
A Bayesian Optimal Design Advancing the Precision Medicine Approach
There is recognized heterogeneity within any given tumor‐type from patient to patient (inter‐patient heterogeneity),...
Read article

July 27, 2021
Thinking of Clinical Development from a Bayesian Lens
Program and portfolio optimization creates a framework throughout the course of the clinical development journey, that...
Read article

July 21, 2021
Bayesian Approach to Benefit-Risk Assessment
Conducting a transparent, targeted and robust benefit-risk assessment of a new drug or product is one of the most...
Read article

July 2, 2021
Use of Rolling-enrollment Designs to Accelerate Clinical Trials
Popular statistical designs, such as CRM (O’Quigley et al., 1990), mTPI-2 (Guo et al., 2017), and i3+3 (Liu et al.,...
Read article

June 25, 2021
Leveraging External Data for Efficient Trial Designs
The main challenge associated with the development of therapies for rare diseases is typically the small study sample...
Read article

June 4, 2021
Bayesian Methods: Paving the path to Clinical Development Transformation
Bayesian methods have been playing a key role in transforming clinical research in therapeutic areas such as oncology...
Read article

June 3, 2021
Information Borrowing in Basket Trials
Bayesian methods allow for the incorporation of prior knowledge, in terms of either expert opinion from clinicians or...
Read article

May 28, 2021
Use of Bayesian Approach in Basket Trial Design
Advancements in biomarkers and momentum in precision medicine has paved the foundation for complex studies like basket...
Read article

May 21, 2021
Use of Bayesian Analysis in Platform Trials: Webinar by Dr. Kyle Wathen
Most of us are familiar with the concept of platform clinical trials as they have gained popularity in the recent...
Read article

May 12, 2021
Strategic Insights from Novel Bayesian Methods – Complimentary Paper
Did you know that Bayesian methods can strengthen Frequentist trials through the use of Bayesian decision criteria or...
Read article
April 23, 2021
Bayesian Methods & Vaccines Research: COVID-19
The urgent need to discover and assess the efficacy and safety of COVID-19 vaccine candidates will affect the future...
Read article

April 14, 2021
Reaping the Benefits of Bayesian Innovation
In the era of modern clinical development, several companies are turning towards novel and innovative approaches such...
Read article
April 2, 2021
Using Bayesian Networks to Predict Survival Outcomes: New Case Study
Earlier this month, my colleagues at Cytel Canada published a paper in JCO Clinical Cancer Informatics, offering a...
Read article

March 26, 2021
Using External Evidence for Decision-Making for Medical Devices
Former Commissioner of the FDA, Dr. Scott Gottlieb, in several public presentations, would bemoan missed chances to...
Read article

March 5, 2021
Single Arm Multi-Stage Phase 2 Cancer Trials
Early stage Phase 2 clinical trials are often designed as multi-stage single arm trials, which quickly identify...
Read article

March 2, 2021
Hybrid Bayesian and Frequentist Clinical Trial Designs
Most people know that clinical drug discovery is usually conducted using either Frequentist or Bayesian methods. These...
Read article
February 26, 2021
Empowering Statisticians to Create Complex Bayesian Clinical Study Designs
In the world of clinical trials, the pace of innovation is accelerating, and approaches such as Bayesian methods are...
Read article

February 18, 2021
Introduction to Evidence Synthesis and Bayesian dynamic borrowing
In the last few years, there has been a growing interest in historical borrowing or augmented trials. There is an...
Read article

February 12, 2021
Leveraging Synthetic and External Control Arms Using Bayesian Methods
In recent times, Single arm trials are being increasingly used to assess new treatment interventions. They establish...
Read article

February 2, 2021
Bayesian Methods for Master Protocols
As the use of master protocols becomes more prevalent in drug development, Bayesian methods are extensively used to...
Read article

January 26, 2021
Cytel Thought Leadership on Power of Bayesian Methods
Bayesian models offer a flexible way of incorporating historical controls in the analysis of trial data (whether single...
Read article

December 17, 2020
2020 Recap by Yannis Jemiai, Chief Scientific Officer, Cytel
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article

December 16, 2020
2020 Recap by Pantelis Vlachos, Principal/Strategic Consultant, Cytel
As we prepare to close the door on 2020, we asked Pantelis Vlachos, Principal/Strategic Consultant for Cytel, to share...
Read article
November 17, 2020
Role of Prediction and Causal Inference in Clinical Research
As a part of Cytel’s "New Horizons Webinar Series", Alind Gupta, Senior Data Scientist, presents case studies from his...
Read article

November 16, 2020
Bayesian Methods for Multiple Cohort Expansion (MuCE) designs
MUCE is a Bayesian solution for cohort expansion trials where multiple dose(s) and multiple indication(s) are tested in...
Read article

October 27, 2020
Bayesian Dose-Finding Designs – An Overview
Cytel recently conducted a webinar on Bayesian Dose-finding Designs for Modern Drug Development, presented by Dr. Yuan...
Read article

October 26, 2020
Need for Technology Solutions to Support Computationally
Pharmaceutical and biotech companies are under pressure to deliver more and deliver faster with fewer resources. The...
Read article

October 22, 2020
An Interview with Bart Heeg (Part 2): New Trends in HEOR
In this two-part blog series, we interview Bart Heeg, Vice President HEOR and Founder at Ingress Health (A Cytel...
Read article

October 12, 2020
Bayesian Statistics and FDA Regulatory Acceptability
Cytel and Novartis are together hosting a complimentary Bayesian Virtual Symposium and an Interactive 7-part workshop....
Read article

October 5, 2020
The Meta-Analytic-Predictive Priors Generation and Comparisons
Staying abreast of the rapid pace of clinical development means adopting innovative or computationally intensive...
Read article

September 30, 2020
A Virtual Event Brought to you by Cytel and Novartis on Innovations
Today, there is a need for advanced quantitative techniques to combine all available information for better decision...
Read article

September 17, 2020
East Alloy: Accelerating the pace of innovation
Keeping up with the rapid pace of clinical development means that we need to adopt the innovative or computationally...
Read article

September 4, 2020
Cytel Co-Founder Cyrus Mehta Presents at the Heart Failure Collaboratory, a Public-Private Partnership with FDA
On Friday September 11, Cyrus Mehta, co-founder of Cytel, will be delivering a talk to the Heart Failure Collaboratory,...
Read article

August 31, 2020
Adopt innovative and computationally intensive designs with East Alloy
Pantelis Vlachos, Principal, Strategic Consultant at Cytel, conducted a webinar to introduce the capabilities of East...
Read article

August 26, 2020
Career Perspectives: Interview with Yannis Jemiai, Chief Scientific Officer
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article

August 19, 2020
Webinar on Adaptive Designs for Dose Finding: Part 2
Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson provided...
Read article

August 13, 2020
Webinar: Adaptive Designs for Dose Finding
Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson provided...
Read article
August 11, 2020
Optimizing Information in Trial Design and Implementation
While there is increasing optimism about the discovery of a COVID-19 vaccine, one of the less talked about aspects of...
Read article

July 27, 2020
Introduction to Population Enrichment by Dr. Thomas Burnett
Cytel is conducting a webinar series on complex innovative trial designs. Dr. Thomas Burnett, Senior Research Associate...
Read article

July 22, 2020
Access Sustainable, Verified Innovation with East Alloy
Cytel brings to you a new blog series on technology and Bayesian decision-making by Pantelis Vlachos,...
Read article

June 24, 2020
Webinar - Practical Model-based Approaches for Phase I Oncology Trials
Last week, Cytel conducted its third webinar in the new introductory webinar series on Complex Innovative Trial...
Read article

June 18, 2020
Why You Should Construct Primary Endpoints Using Bayesian Methods
One of the revelations of the COVID-19 pandemic is that the flexibility and potential of Bayesian designs goes far...
Read article

June 15, 2020
Significance of Bayesian Model-Based Approaches in Oncology Trials: An Interview with Dr. Satrajit Roychoudhury
Cytel conducted a webinar with Dr. Satrajit Roychoudhury, Senior Director, Statistical Research and Data Science...
Read article
June 11, 2020
Adaptive Bayesian Methods: The Secret Weapon in COVID-19 Vaccine Development
A recent Cytel panel led by Vice President of Strategic Consulting Natalia Muhlemann evaluated the role that Bayesian...
Read article
April 29, 2020
Webinar: Transparent Machine Learning in Oncology
In our previous blog, we spoke with Alind Gupta, who works as a Machine Learning Researcher at Cytel in Canada. The...
Read article

April 20, 2020
Interview with Alind Gupta: Transparent Machine Learning in Oncology
Cytel is hosting a webinar on Transparent Machine Learning in Oncology, on April 21, 2020. Our speaker, Alind Gupta,...
Read article
January 30, 2020
Designing Event-based Studies: Interview with Pantelis Vlachos
The Cytel Trial Design Innovations (CTDI) Webinar Series recently hosted a webinar on designing event-based studies....
Read article

January 16, 2020
Adaptive Population Enrichment in a Phase III Oncology Trial
January’s Cytel Trial Design Innovations (CTDI) Webinar Series will feature Biostatistician and pioneering Bayesian...
Read article

November 12, 2018
Can Statisticians Contribute to Enhance the Position of Patients in Clinical Trials?
In this blog, we talk with Robert Greene, Founder and President of the HungerNDThirst Foundation, about his upcoming...
Read article

October 5, 2018
Interview with Stephen Senn: 70 Years and Still Here: The Randomized Clinical Trial and its Critics
We are delighted that Stephen Senn will be joining us at the EUGM on November 14th and 15th in Darmstadt, Germany. In...
Read article

September 27, 2018
Decision Making in Development Programs with Targeted Therapies: with Heiko Götte
In this blog, we talk with Heiko Götte, Senior Expert Biostatistician at Merck about his upcoming presentation at...
Read article

August 15, 2018
2018 East User Group Meeting Addresses Multiplicity Themes, with keynotes including Stephen Senn and Meinhard Keiser.
Cytel’s 7th East User Group Meeting (EUGM) will take place on November 14 & 15, 2018 at Merck in Darmstadt, Germany,...
Read article

May 30, 2017
Interview: How can a Bayesian framework support benefit risk assessment?
A recent paper The case for Bayesian methods in benefit-risk assessment: Overview and future directions (1) co-authored...
Read article
March 24, 2017
Case Study: Improving Go/No-go Decision-Making with Custom Software
Robust go/no-go (GNG) decision-making is essential for effectively managing risk across a clinical portfolio. In early...
Read article

March 2, 2017
Case Study: Bayesian Decision-Making in a Phase 3 Oncology Design
We continue our case study series with this example of a Phase 3 design that uses Bayesian decision making combined...
Read article

July 21, 2016
Webinar Replay: Single and Double Agent Dose Escalation Designs
Did you miss our webinar on Single and Dual Agent Dose escalation designs earlier in the year? In this blog we have...
Read article

May 31, 2016
EAST 6.4 Release: Interview with Yannis Jemiai
Last week, we were delighted to announce the release of East 6.4 bringing further cutting –edge approaches to the East...
Read article
May 19, 2016
Pattern Recognition and 'Big Data'
The explosion in healthcare information and “big data “has been one of the most written about topics in the last few...
Read article

March 29, 2016
Decision Making in Early Clinical Development
On March 16th and 17th the 5th East User Group Meeting took place in London. This very successful 2 days saw a variety...
Read article
March 23, 2016
What's the Value of P?
“How many statisticians does it take to ensure at least a 50% chance of a disagreement about p-values?” So questions...
Read article
November 12, 2015
2 Talks on Early Phase Go/No-GO Decision Making
Last week Cytel joined forces with Sanofi/Genzyme to devote a full day of workshops and talks related to modern methods...
Read article
November 10, 2015
Bayesian Dose Escalation Designs for Late Onset Toxicity
Last week Cytel joined forces with Sanofi/Genzyme to devote a full day of workshops and talks related to modern methods...
Read article
October 27, 2015
P-Values & Pharma Development: We Want to Hear from You
Here at Cytel we have enjoyed following the debates on the p-value controversy currently taking place on the ASA...
Read article

September 1, 2015
Modern Early Phase Clinical Trial Design Primer
If you’re in the practice of conducting early phase clinical trials, you’ve probably heard that modern trial designs...
Read article
March 20, 2015
How Bayesian Strategies Can Expedite a Pediatric Clincial Trial Time by 20 - 40%
Sometimes a new candidate drug for a pediatric study has already been tested on adults for safety and efficacy. We know...
Read article
February 17, 2015
How Proposed Regulatory Reforms Will Affect Your Clinical Trial
21st Century Cures (also called Cures2015) is a bipartisan initiative undertaken by the Committee on Energy and...
Read article
January 29, 2015
Adaptive Design and Bayesian Statistics: 5 Years Later (Podcast)
February 2015 marks the five year anniversary of the FDA’s Guidance on Adaptive Design Clinical Trials for Drug and...
Read article
December 9, 2014
Drug Supply Planning for Dose-Ranging Adaptive Trials
When planning a conventional trial, one can anticipate the drug supply necessary for the trial by determining how the...
Read article
November 18, 2014
A Bayesian Industry Approach to Phase I Combination Trials in Oncology
Statisticians and scientists at Novartis have been at the forefront of developing a new method in early phase oncology...
Read article
October 2, 2014
Bayesian-Bandit Adaptive Designs for Rare Disease Drug Development
Sofia S. Villar is a member of the DART (Design and Analysis of Randomised Trials) group at the MRC Biostatistics Unit...
Read article
July 31, 2014
Bayesian Approaches in Clinical Trials: Updates on Tools & Techniques
Statisticians at Cytel are staunch advocates of the use of Bayesian methods in clinical trials. This summer's Joint...
Read article
July 15, 2014
Cytel Animation: Modern Dose Escalation Phase 1 Trials
Do you design or analyze Phase 1 dose escalation trials? Have you considered methods other than 3+3? This new Cytel...
Read article

May 19, 2014
Frequentist? Time for an update!
Pantelis Vlachos, PhD, is a Director at Cytel Consulting. He works with a team of experts who regularly assist clinical...
Read article
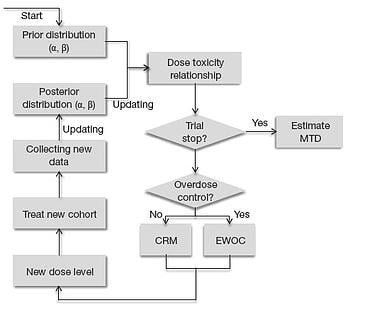
May 8, 2014
5 Reasons to Invest in Bayesian Dose-Escalation Methods
( Editor's note: This post has been refreshed in December 2016) Model based algorithms for Phase I dose-escalation have...
Read article


