Introduction to Population Enrichment by Dr. Thomas Burnett

Cytel is conducting a webinar series on complex innovative trial designs. Dr. Thomas Burnett, Senior Research Associate in Medical and Pharmaceutical Statistics at Lancaster University, joined us as the presenter in the latest webinar from this series. In this webinar, “Adaptive Enrichment Designs in Clinical Development”, Dr. Burnett provides us a brief introduction to population enrichment and explains where it fits in clinical trials. He offers his insights on the topics of hypothesis testing and decision making, which is a key component of adaptive designs. You can also learn about a real-world case study (TAPPAS Trial) where this approach was used. Continue reading this blog for highlights from the webinar.
Watch the webinar recording and download the slides by clicking the button.
Read an interview with Dr. Thomas Burnett on adaptive enrichment.
What is population enrichment?
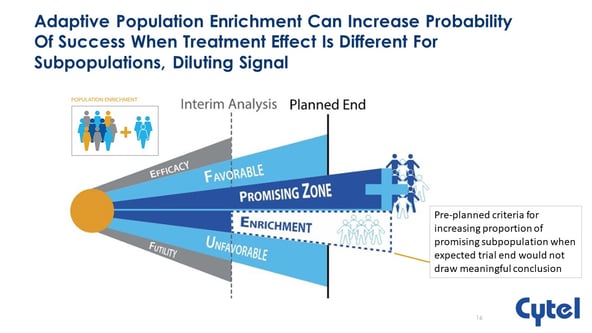
For any clinical trial where you are developing a new treatment, we have a target population or there can be a subset of those people who have certain characteristics that you are in interested in, for the purpose of the trial. Within the classical setting, we randomize patients under experimental treatment and control treatment, and this allows us to test the null hypothesis. We may have sub-groups in this population that we expect to interact differently with the treatment that we are applying. The key point we use for adaptive enrichment is that these sub-populations are predefined. Which means we know about their existence before the trial begins.
There are different ways in which we can define our null hypothesis. We can make our examinations in these different subgroups or alternatively, test in the full population, that is the combination of the two subgroups. The sampling for the design is set up at the start of the trial, and after we run all the way through the trial, at the end of that sampling we have our analysis. We could conduct the trial in just a single population, in this case population 1. Using the same amount of resources, that is the same number of patients, we could conduct the trial in population 2. The third option is to use the full population. Option 4 is to recruit everybody and test within subgroups. In a classical setting our question is which patient group to recruit and therefore study in the trial? There might be different opinions within the trial team. Population enrichment design helps us resolve this dilemma. We begin by recruiting every patient we can into the trial or some portion of the total sample size and randomize those patients to compare experimental treatment and control treatment. At this stage we decide how to recruit patients for the remainder of the trial. We choose from population 1, 2 and 3 based on the observations of stage 1 of the trial. Dr. Burnett explains the factors we need to consider for decision making. For example, there should not be a long delay between recruitment of a patient and the observation of the primary endpoint.
Population enrichment method allows us make data driven decisions at the interim analysis. We can apply these methods in multiple data types and multiple settings.
Hypothesis Testing
We have null hypothesis in each sub-group and the combined population group, and we study those over multiple stages. In a typical single hypothesis trial, you will be concerned with falsely declaring efficacy, or making a type I error. In a multiple hypothesis setting, we are still concerned with that same essential problem of falsely declaring efficacy but because we have multiple arms, we state the error as FamilyWise Error. In a confirmatory trial setting we would be concerned with controlling the error rate at some prespecified level and this is known as strong control of familywise error. A critical fact that we lean on to ensure we achieve strong control is that we have preplanned trial options. Thus, we can continue recruitment in population 1, 2 or 3. What we need is a testing procedure that will cover each of these possible eventualities of the trial in terms of controlling the error rate. Dr. Burnett explains how to achieve a closed testing procedure.
Decision Making
We make decisions based on the available data or information from stage I where we recruit from all populations. We get estimates of the treatment effect after the randomization. These estimates help our decision making. Dr. Burnett talks about Bayesian decision making and adaptive enrichment which were his subjects for his PhD thesis. The crucial difference in this approach is the capturing of prior uncertainty about the treatment effect before the trial. You can use Bayesian decision making without impacting the hypothesis testing of the trial.
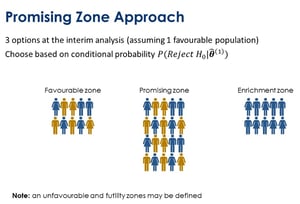 In the webinar, he also explains the promising zone approach. Here, we look at three options at the interim analysis, assuming one of the populations is favorable for the treatment. The idea is that we will either enrich into that population and thereby, continue recruiting only from that sub population. We also might be in a favorable zone where we continue the trial as is or we may be in a promising zone where we might choose to recruit more patients. In the promising zone we can review our sample size for the trial moving forward. This is the approach that is implemented in the ENRICH module of East.
In the webinar, he also explains the promising zone approach. Here, we look at three options at the interim analysis, assuming one of the populations is favorable for the treatment. The idea is that we will either enrich into that population and thereby, continue recruiting only from that sub population. We also might be in a favorable zone where we continue the trial as is or we may be in a promising zone where we might choose to recruit more patients. In the promising zone we can review our sample size for the trial moving forward. This is the approach that is implemented in the ENRICH module of East.
TAPPAS Trial
This trial makes use of the promising zone approach. It was a study of TRC105 in patients with advanced angiosarcoma We were able to identify two sub-groups before the trial began. These were the cutaneous advanced angiosarcoma sub-group and the non-cutaneous advanced angiosarcoma. The Indication in this case was of greater tumor sensitivity in cutaneous sub-group. This is exactly the kind of setting where we would like to apply adaptive enrichment. The flexibility in this case was useful as it helped us find a particularly strong effect within a subgroup where we were able to focus our attention.
To watch the complete webinar and download the slides, click on the button.
Join Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson in the next webinar in the series.
Finding the right dose in Phase 2 gives a potential new therapy its best chance to demonstrate efficacy during Phase 3. A well-executed dose-ranging trial therefore has the potential to alter the course of the entire clinical development program. This webinar will demonstrate how adaptive and Bayesian techniques can be implemented for optimal dose-finding.
Adaptive Designs for Dose-Finding
August 5, 2020 8am (PST) | 11am (EDT) | 5pm (CET)
About Thomas Burnett
 Thomas is a Senior Research Associate in Medical and Pharmaceutical Statistics at Lancaster University, where his main research interests are Adaptive clinical trials and personalised medicine. He studied for his BSc in Mathematics and Statistics with placement at the University of Bath, spending a year working on sample design at the Office for National Statistics. Thomas also holds a PhD from the University of Bath, this was a case studentship that involved working closely with Roche Products Ltd researching the Bayesian optimisation of Adaptive Enrichment trials.
Thomas is a Senior Research Associate in Medical and Pharmaceutical Statistics at Lancaster University, where his main research interests are Adaptive clinical trials and personalised medicine. He studied for his BSc in Mathematics and Statistics with placement at the University of Bath, spending a year working on sample design at the Office for National Statistics. Thomas also holds a PhD from the University of Bath, this was a case studentship that involved working closely with Roche Products Ltd researching the Bayesian optimisation of Adaptive Enrichment trials.



