
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

February 23, 2024
Written by Angelo Tinazzi, Nicolas Rouillé, and Sebastià Barceló In the realm of standards management, companies of all...
Read article

January 3, 2024
New FDA Data Submission Requirements and Substantial Changes
Ten years ago this month, in January 2014, the FDA issued the first version of its Technical Conformance Guide (by...
Read article

December 27, 2023
Top Data Submission and Data Integration Posts of 2023
Perspectives covers a wide range of topics related to data submission and data integration, from ISS and ISE best...
Read article

December 8, 2023
Discussions with the FDA and Ensuring Data Submission Success
Regular technical discussions with the FDA play a critical role in ensuring data submission success. These discussions...
Read article

November 1, 2023
The Changing Landscape of the Pharmaceutical Industry: A Preview of Cytel’s Contributions at PHUSE EU 2023
It feels like just yesterday I attended my first PHUSE conference back in 2005 in Heidelberg, Germany. Fast forward 19...
Read article

October 23, 2023
Experiencing the CBER: Anticipating Unique Challenges
The Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) are the...
Read article

August 25, 2023
Preparing and Concluding Your FDA Data Submission, and More Insights on Data Submission and Data Integration
For several years, CDISC and Regulatory Data Submission expert Angelo Tinazzi has authored the series, The Good Data...
Read article

August 14, 2023
The Evolution of Open-Source Initiatives and New Standards Development for the Data Submission of the Future
In the first part of this post, I discussed the ongoing revolution, or maybe I should say evolution, we are living...
Read article

August 9, 2023
Standards and Open Source Hand-in-Hand: Leveraging Automation to Expedite Drug Market Request Review Process
How do you envision the future of data submission? Last week, I had the privilege of presenting the topic “Standards...
Read article

June 2, 2023
Presenting Clinical Data for Regulatory Submission: A Stats Perspective
Data submissions are very regulated, but every drug and drug development are different. Therefore, the data presented...
Read article

May 24, 2023
It’s Time to Move, Time to Move to Define-XML 2.1
As of March 2023, specifically for any study started on or after March 15, 2023,1 for the submission of SEND, SDTM, and...
Read article

April 26, 2023
New Ebook: “The Good Data Doctor on Data Submission and Data Integration”
Regular technical discussions with the FDA play a critical role in ensuring data submission success. These discussions...
Read article

April 21, 2023
CDISC Europe 2023: A Preview
It was early March 2020, after the world was hit by the Covid-19 pandemic, that those of us on the CDISC Eu committee...
Read article

February 8, 2023
The Facts in the Case of Subject X
Over the past years, probably the entire last decade, there have been several discussions on how to handle multiple...
Read article

December 9, 2022
(Re)Integration Dilemma: Integrated Summaries of Safety and Effectiveness
As promised in my last post prior to PHUSE-EU Connect, I’d like to now share some reflections on my “Integration...
Read article

November 8, 2022
A Preview of Cytel’s Contributions to PHUSE EU 2022
Although many of you can’t wait for the start of the Football World Cup 2022 (less than two weeks while I’m writing),...
Read article

September 16, 2022
Raising Awareness for Additional FDA Data Standards Submission Recommendations (Part II)
In the first part of this article, I raised awareness of the availability of additional FDA guidances containing CDISC...
Read article

September 2, 2022
Summer Weekend Read Roundup
Last week, we featured our final Summer Weekend Read, the last in a series designed to showcase some of our most recent...
Read article


June 15, 2022
Raising Awareness for FDA Data Submission Recommendations (I)
For years CDISC data standards implementers have struggled to find good implementation examples and use cases beside...
Read article

April 25, 2022
Insights on the New ADaM guidelines and Europe Interchange 2022
I am excited to see you all at the CDISC Europe Interchange, April 27 – 28 but unfortunately, it will be a virtual...
Read article

February 11, 2022
WINTER WEEKEND READ: Tops Tips and Tricks from the Good Data Doctor
Adopting data standards such as CDISC in the early phase of clinical drug development contributes to the consolidation...
Read article

January 25, 2022
Watch out, the FDA Rejection Criteria are Now in Place
In this blog, I share some experiences we recently had during an FDA submission Cytel performed for a sponsor after...
Read article

December 23, 2021
Year-End Roundup: Your Favorite Blog Posts of 2021
Cytel blogs bring you debate and discussion of the newest trends in statistics and quantitative strategy. In 2021, our...
Read article

December 15, 2021
CDISC SDTM and ADaM: An Explosive 2021 Ending!
Recently, on November 29 I received an email from CDISC announcing an important update for both SDTM and ADaM CDISC...
Read article

November 30, 2021
The FDA “Real-Time Oncology Review” Process
The FDA “Real-Time Oncology Review (RTOR)”[1] is an “FDA project started in 2018 to facilitate earlier submission of...
Read article

October 5, 2021
The Importance of Traceability
Traceability is crucial in all steps of clinical data handling, from data collection to final analysis. The importance...
Read article

August 31, 2021
CDISC Certification - is it worth taking?
For years, I have been telling the recruiters at Cytel to be wary of candidates claiming to have a CDISC Certification...
Read article

July 30, 2021
In a Virtual Room with the FDA Reviewers
I had recently (for the first time) the pleasure and honor to attend a virtual meeting with the FDA, a pre-NDA Type-B...
Read article
April 26, 2021
Why you should not miss 2021 Virtual CDISC EU Interchange?
As we all continue to take necessary precautions against the spread of COVID-19 virus, this year again the CDISC EU...
Read article

March 30, 2021
The Integration Dilemma
As of today, our Industry has not defined any approach, nor does an official regulatory agency...
Read article

February 24, 2021
Avoiding Lost-in-Translation with Submission Terminology
In a previous post, I discussed the importance of proper use of CDISC Controlled Terminology (CDISC CT) in SDTM....
Read article

January 28, 2021
A little walk in the CDISC Library, hand in hand with SAS
The Christmas break presented an opportunity to make my first concrete steps into the CDISC Library. Overall, it was a...
Read article

December 21, 2020
Year-End Roundup: Your Favorite Blog Posts of 2020
2020 has been an unusually difficult year as the global pandemic impacted all of our lives. This year, the Cytel blog...
Read article

September 14, 2020
From Before to After: Preparing and Concluding your FDA Data Submission
“A good start is half the battle” (the Before) when submitting data to the FDA and there are a couple of cherries to...
Read article

July 28, 2020
Therapeutic Area User Guidance – The hidden Gems
CDISC standards have been around for a while with the first SDTM Standard version released in 2004. However, it was...
Read article

June 29, 2020
The Good Data Submission Doctor: CDISC for COVID-19
From the time the COVID-19 outbreak was declared a pandemic, the number of studies conducted around the world to either...
Read article

April 27, 2020
Highlights from the 2020 Virtual CDISC EU Interchange - Part 2
In the first part of this two-parts blog, I speak about how the European CDISC Committee (E3C) together with CDISC...
Read article

April 24, 2020
Highlights from the 2020 Virtual CDISC EU Interchange by Angelo Tinazzi
In early March, when countries around the world started implementing lockdowns, the European CDISC Committee (E3C)...
Read article

December 18, 2019
Year-End Roundup: Your Favorite Blog Posts of 2019
With only two weeks left for this fabulous year to end, we would like to thank all our blog subscribers and new readers...
Read article

October 23, 2019
The Good Data Submission Doctor - New ADaM Implementation Guidance
October 3, 2019 was an important day for the ADaM team as it marked the release of the ADaM Implementation Guidance...
Read article

June 27, 2019
Handling the specialized data requirements in oncology clinical trials
By Nicolas Rouillé and Eric Henniger The right design and the right data ultimately leads to the right decisions, so...
Read article



November 27, 2018
Top 5 Frequently Asked ADaM Questions
This is the third in our blog series ' The Good Data Submission Doctor' in which Angelo Tinazzi, Director of Standards,...
Read article

November 1, 2018
Top 5 Frequently Asked SDTM Questions
In this second post of the “Good Data Submission Doctor” ( read my first post The Master Recipe: Quality and Attention...
Read article


October 1, 2018
Details Matter When Submitting CDISC Packages to Authorities
One of my wife’s favorite TV shows is ‘Quattro Ristoranti’ (Four Restaurants). In each episode of the show, 4...
Read article

January 9, 2018
Career Perspectives: Interview with Lisa Goldberg, Associate Director of Statistical Programming
Our Career Perspectives' series is back! Cytel has industry-leading experts in statistical programming with years of...
Read article
June 28, 2017
Under wraps: the importance of patient privacy
About the Author: Manjusha Gode has over 28 years' IT experience spanning delivery Management, quality management,...
Read article

May 17, 2017
Case Study: From Trial Design to CDISC Submission
This new case study shares how Cytel supported a specialist biopharmaceutical company from Phase 2 trial design through...
Read article

February 6, 2017
The Making of a CDISC Trainer
CDISC is a global, nonprofit charitable organization whose mission is ‘to inform patient care and safety through higher...
Read article

December 21, 2016
CDISC submissions- are you up to speed?
December 18th 2016 was a significant date for the pharmaceutical industry and regulatory submissions. For trials which...
Read article

December 20, 2016
How do CDASH standards build data quality?
Data Standards play a crucial role in structuring and promoting long term value of clinical data. Clinical Data...
Read article
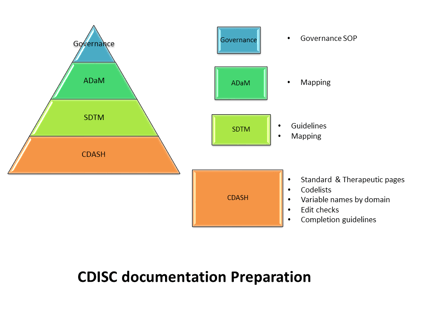
August 2, 2016
The CRO role in Data Standards Governance
Editor's note( this blog was refreshed in April 2018) As CDISC compliant submissions become increasingly expected,...
Read article

July 25, 2016
5 trends a statistical programmer needs to follow
Statistical programmers are in high demand within the biopharmaceutical industry, and within the dynamic world of...
Read article

May 12, 2016
Lost in Traceability- From SDTM to ADaM
Once upon a time Hansel and Gretel laid a trail of breadcrumbs which they followed to find their way back home. Their...
Read article

March 8, 2016
Mind the Gap! How to prepare for SDTM migrations.
Data standardization is critical to ensure successful regulatory submissions. While many sponsors now choose to create...
Read article


