Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

April 12, 2024
How should health technology developers prepare for future market access activities in Europe? Numerous discussions...
Read article

March 20, 2024
Key Elements and Implications of the Draft EU JCA Implementing Act
Written by Lydia Vinals, PhD, and Grammati Sarri, PhD The draft Implementing Act of the EU Health Technology Assessment...
Read article

December 20, 2023
Top Real-World Evidence and Real-World Data Topics of 2023
Perspectives covers a wide range of topics related to real-world evidence and real-world data, from overcoming health...
Read article

December 1, 2023
Leveraging Real-World Data for Comparison Using Synthetic Control Arms
Last week, Robert Szulkin, Research Principal, Real World Evidence, discussed the need for real-world evidence studies,...
Read article

November 3, 2023
Reinforcement Learning: A Promising Tool for Predicting Optimal Treatment in Complex Diseases
Written by Fei Tang and Evie Merinopoulou Reinforcement Learning (RL), a crucial component of machine learning (ML),...
Read article

October 25, 2023
Unravelling PICO: The Pillars of the European Joint Clinical Assessment
The European Union (EU) health technology assessment (HTA) regulation aims to improve the availability of innovative...
Read article
October 20, 2023
Mind the Health Gap: Is It Time to Reliably Measure the Impact of Health Inequity in Product Development and Assessment? Yes, It Is.
Written by Grammati Sarri and Yannis Jemiai The spotlight for this year’s World Evidence-Based Healthcare Day (EBHC)...
Read article

October 2, 2023
A Better Way to Track Medication Adherence
Patients’ adherence to the medications or treatment regimens prescribed to them by their clinicians is an important...
Read article

September 8, 2023
Synthetic Control Arms: Leveraging Real-World Data for Comparison in Single-Arm Trials
Single-arm trials, unlike placebo-controlled randomized control trials, forgo the use of a placebo or standard-of-care...
Read article

July 28, 2023
Embracing AI and ML in Medical Devices: FDA’s Total Product Lifecycle-Based Regulatory Framework
Written by Fei Tang, RWE Senior Research Consultant, and Paul Arora, Assistant Professor (Status), Dalla Lana School of...
Read article

July 14, 2023
Real-Life Data-Sharing and EU Joint Clinical Assessments: Is Closing this Chasm a Mission Impossible?
Written by Grammati Sarri, David Smalbrugge, Andreas Freitag, and Evie Merinopoulou The vision of a single, centralized...
Read article

May 22, 2023
New CADTH Guidance on RWE Is Now Available, but Critical Aspects Are Still Missing
By Grammati Sarri, Evie Merinopoulou, Vinusha Kalatharan, and Jason Simeone The Canadian Agency for Drugs and...
Read article

May 10, 2023
FDA Increases Calls for Manufacturers to Ensure Trial Diversity, but Does It Fall Short of Addressing Health Inequalities in Product Development?
The evidence is staggering on the unequal health burdens experienced by specific patient groups defined by ethnic,...
Read article

March 21, 2023
Retrospective Claims Data Analysis Unlocks Discovery in Multiple Sclerosis Research
One of the lesser-known complications associated with Multiple Sclerosis is a higher risk of serious infections (SIs)....
Read article
March 8, 2023
New Linked Database Expands Potential of RWE: A Unique Opportunity for Multiple Sclerosis Research
Real-world data has been increasingly used to answer questions related to the course, prognosis, and treatment of...
Read article
March 7, 2023
Can RWE Help Restore Decades of Health Inequalities? Yes, and Here’s How
Health inequalities are an enduring issue that can be exacerbated by clinical trial recruitment that does not reflect...
Read article

February 14, 2023
FDA Guidance on the Design and Conduct of Externally Controlled Trials — What to Watch
The U.S. FDA has recently provided specific guidance[i] on the design and conduct of trials incorporating an external...
Read article

January 31, 2023
Linked Data Studies: Improving the Way We Do Observational Research in Germany
Asthma affects more than 235 million people worldwide, and due to lacking effective implementation of clinical...
Read article

October 4, 2022
MCMC vs. INLA in Bayesian Adaptive Clinical Trial Designs
Integrated Nested Laplacian Approximations (or INLA) are now starting to be used by statisticians as a key tool for...
Read article

May 13, 2021
An Interview with Radek Wasiak, Head of Real World and Advanced Analytics at Cytel
Cytel’s HEOR and RWE Expertise has grown quite significantly in the past year. Could you speak a little bit about the...
Read article
May 5, 2021
Meet our Global HEOR and RWE Experts: Virtual ISPOR 2021
Cytel’s team of HEOR and Real-World Evidence (RWE) experts are all set to make a splash at the Virtual ISPOR on May 17...
Read article

April 21, 2021
Understanding Head-to-Head Clinical Trials
Two therapies are placed in head-to-head clinical trials when they are compared against each other as opposed to a...
Read article

April 7, 2021
Career Perspectives: Interview with Neha Sati
Scientists at Cytel recently published a paper in the Journal of the American Medical Association (JAMA). Among the...
Read article
April 2, 2021
Using Bayesian Networks to Predict Survival Outcomes: New Case Study
Earlier this month, my colleagues at Cytel Canada published a paper in JCO Clinical Cancer Informatics, offering a...
Read article

February 9, 2021
New Meta-Analysis in JAMA Uses Novel Quantitative Techniques to Demonstrate Baseline Characteristics Informing Response to Common Therapy for Kidney Cancer
Recent years have witnessed improving survival outcomes for those struggling with a range of common kidney cancers....
Read article

January 22, 2021
The Role of Real World Evidence after COVID19
COVID-19 has transformed the pharmaceutical industry in a manner that few could have predicted only a year ago. One of...
Read article

December 17, 2020
2020 Recap by Yannis Jemiai, Chief Scientific Officer, Cytel
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article

November 24, 2020
Cytel and Ingress Health at Virtual ISPOR Europe 2020
Virtual ISPOR 2020, held November 16 to 19, presented new opportunities for scientific interaction amongst HEOR...
Read article
November 17, 2020
Role of Prediction and Causal Inference in Clinical Research
As a part of Cytel’s "New Horizons Webinar Series", Alind Gupta, Senior Data Scientist, presents case studies from his...
Read article

November 12, 2020
Join Cytel and Ingress Health at Virtual ISPOR Europe 2020
Cytel and Ingress Health (now a Cytel company) will be contributing to a range of events at Virtual ISPOR EUROPE 2020,...
Read article
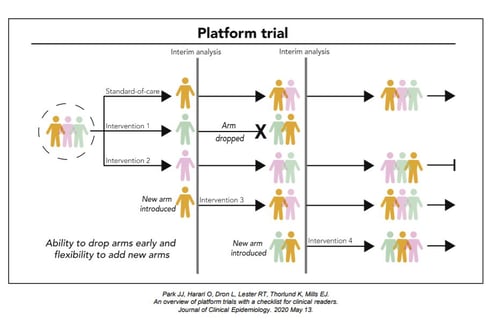
November 10, 2020
Key Design Considerations for Platform Trials
Platform trials are a new type of clinical trials where multiple interventions can be evaluated simultaneously against...
Read article

November 5, 2020
Role of RWA in Transforming Oncological Research
In oncology, many manufacturers go into niche indications, where there are very specific tumors, and then they opt for...
Read article

October 22, 2020
An Interview with Bart Heeg (Part 2): New Trends in HEOR
In this two-part blog series, we interview Bart Heeg, Vice President HEOR and Founder at Ingress Health (A Cytel...
Read article

October 20, 2020
Interview with Thomas Wilke: Health Economics/World Evidence Studies
In this interview with Thomas Wilke, Principal Scientist at Ingress-Health (a Cytel company), we talk to him about his...
Read article

October 19, 2020
The Uniqueness of COVID-19 Data Challenges; The COVID-19 trial tracker
COVID-19 has created extreme uncertainties -- a dearth of historical information combined with the need for safety,...
Read article

October 14, 2020
The Increasing Importance of Health Economics
A credible evidence base is needed to support and document the economic value of new technologies and therapeutic...
Read article

September 22, 2020
LIBERTY claim analysis manuscript by Thomas Wilke and Sabrina Mueller
Research Scientists, Thomas Wilke and Sabrina Mueller recently published a manuscript on “Diabetes-Related...
Read article

August 26, 2020
Career Perspectives: Interview with Yannis Jemiai, Chief Scientific Officer
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article

August 5, 2020
Design and Data Considerations from Cardiovascular Pilot Investigation
Cytel is conducting two pilot projects on head-to-head comparisons using real world data. These projects in oncology...
Read article

July 23, 2020
Why Consider a Synthetic or External Control Arm?
Just as there are numerous adaptations that fall within the umbrella of adaptive designs, there are several different...
Read article

July 21, 2020
Head to Head Comparisons Using Real World Data
Cytel is conducting a webinar series that focuses on target trial emulation and causal inference approaches using real...
Read article
June 23, 2020
Design and Data Source Considerations from Pilot Investigations in CVD
Supposing two treatments, A and B, need to be compared that have not been compared through a clinical trial. In the...
Read article
April 29, 2020
Webinar: Transparent Machine Learning in Oncology
In our previous blog, we spoke with Alind Gupta, who works as a Machine Learning Researcher at Cytel in Canada. The...
Read article

April 20, 2020
Interview with Alind Gupta: Transparent Machine Learning in Oncology
Cytel is hosting a webinar on Transparent Machine Learning in Oncology, on April 21, 2020. Our speaker, Alind Gupta,...
Read article
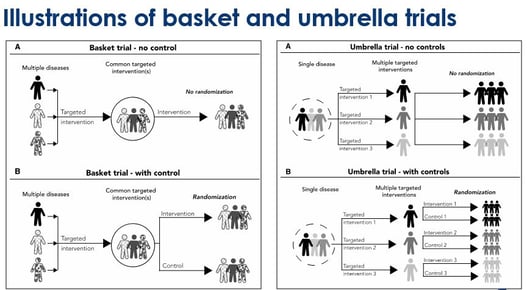
March 31, 2020
Key Design Thoughts for Basket Trials and Umbrella Trials by Jay Park
Since 1953, when the discovery of the structure of DNA was made, we have seen great advancements in genomics....
Read article
March 26, 2020
Cytel's Response: EMA Points to consider on implications of COVID-19
Further regulatory guidance has been released concerning the implications of the Coronavirus disease (COVID-19) on...
Read article

March 23, 2020
Cytel's Response: EMA Guidance on the Management of Clinical Trials During the COVID-19 (Coronavirus) Pandemic
On March 20th the European Commission, the European Medicines Agency (EMA) and the Heads of Medicines Agency (HMA)...
Read article

March 19, 2020
Cytel's Response: FDA Guidance on Conduct of Clinical Trials during the COVID-19 Pandemic
The FDA issued a guidance yesterday on how the COVID-19 Pandemic may affect the conduct of clinical trials. Below are...
Read article

March 12, 2020
Interview with Jay Park: The present and future of Master Protocols
In September 2018, the FDA provided a draft guidance on master protocols reflecting an increased interest in these...
Read article
June 11, 2015
Aligning Clinical Development & Regulatory Objectives for Cardiovascular Outcome Trials
When the FDA first began to require pharmaceuticals to perform cardiovascular outcome trials to establish the safety of...
Read article


