
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

February 22, 2023
Simulation-guided design is quickly becoming a novel feature of modern drug development. Its foundational promise is to...
Read article

February 9, 2023
Supercharging Quantitative Decision-Making with Simulation-Guided Trial Design
Those familiar with simulation-guided design (SGD) know that it can be used for a wealth of clinical trial options:...
Read article

January 23, 2023
Design Considerations for Early Phase Trials of Immuno-oncology Drugs
Ever since the first immune checkpoint inhibitor was approved for market nearly twelve years ago, the industry has...
Read article

October 28, 2022
Measuring Robustness of Clinical Trial Designs with Pressure Tests
Integrating the “pressure testing” of clinical trial designs into the process of creating a strong clinical trial...
Read article

October 24, 2022
Cyrus Mehta on the Founding of Cytel
On the occasion of Cytel’s 35th anniversary, co-founder Professor Cyrus Mehta sits down with Dr. Esha Senchaudhuri to...
Read article

October 18, 2022
Nitin Patel on 35 Years of Technological Innovation
On the occasion of Cytel’s 35th anniversary, co-founder Professor Nitin Patel sits down with Dr. Esha Senchaudhuri to...
Read article

October 11, 2022
Joshua Schultz on the Evolution of Cytel
On the occasion of Cytel’s 35th anniversary, our CEO Joshua Schultz sits down with Dr. Esha Senchaudhuri to discuss the...
Read article


September 27, 2022
Data Capture and Data Sharing During the COVID-19 Pandemic
On Louis Dron et al., “Data Capture and Sharing in the COVID-19 Pandemic: A Cause for Concern,” The Lancet 4 (10) (2022)
Read article

August 30, 2022
Understanding the Economic Benefits of Platform Trials
Many thanks to Kyle Wathen and Behnam Sharif for their input on this post.
Read article

August 3, 2022
The Uses of Bayesian Methods in Late-Phase Clinical Trial Strategy
A number of late-phase clinical trial sponsors remain hesitant to employ Bayesian approaches in confirmatory settings,...
Read article
July 28, 2022
New Directions in Indirect Treatment Comparisons
When new treatments are compared with existing therapies in clinical care, population-adjustment techniques need to...
Read article

July 26, 2022
7 Ways RWD Is Transforming Clinical Research
To watch this webinar and others from this introductory series, click the link below. The ability to draw on electronic...
Read article

July 20, 2022
The Case for Network Meta-Interpolation to Handle Effect Modifiers in Indirect Treatment Comparisons
When performing indirect treatment comparisons, effect modification can create complexities in the event of high...
Read article

July 13, 2022
5 Steps to Adjust for Effect Modifiers for Treatment Comparisons
Many thanks to Grammati Sarri and Michael Groff for their comments in developing this blog. An indirect treatment...
Read article

July 12, 2022
Strategies for Selecting New Indications for a Platform Trial
Thanks to Dr. Kyle Wathen for comments on this blog. The increasing use of platform trials for the testing of a wide...
Read article

June 29, 2022
Platform Trials, Master Protocols, and Challenges in Execution
How can we build an efficient statistical protocol for a clinical trial, if we do not know the therapies that will be...
Read article
June 14, 2022
6 Key Trials for Understanding Adaptive Designs for Clinical Trials
Suppose you had to choose six clinical trials intended for registration with regulatory agencies, only six, to explain...
Read article
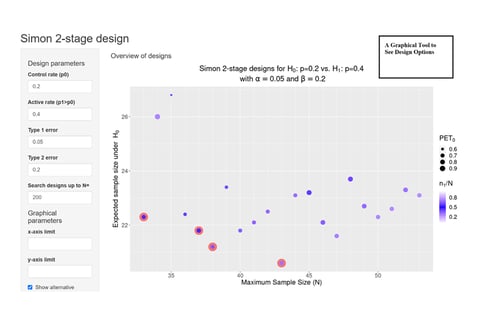
June 8, 2022
On Kappler's 'Graphical Comparison of Simon two-stage designs'
Clinical researchers, seeking to understand the statistical benefits of a common Phase 2 oncology design, now have a...
Read article
June 7, 2022
Continuous Monitoring for Blinded Sample Size Reestimation
In most instances of blinded sample size re-estimation, the timing of the interim analysis that determines whether the...
Read article

June 2, 2022
Digital Transformation for Clinical Trials
How can clinicians at the forefront of modern clinical trials and statisticians at the forefront of advanced...
Read article

May 27, 2022
How to Use a Living HTA Approach to Demonstrate Value in Real-Time
When submitting systematic literature reviews to a Health Technology Assessment authority, high volumes of research...
Read article

May 26, 2022
Method of Estimation with application to the COVID-19 Pandemic
When constructing estimands a key question that arises is how to handle intercurrent events and missing data. In a...
Read article
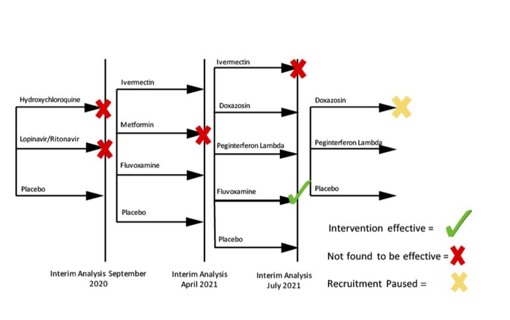
May 17, 2022
Society of Clinical Trials names TOGETHER "Trial of the Year"
Early in the pandemic, it became clear that many of the COVID-19 therapies being tested in wealthier nations, were not...
Read article

May 16, 2022
Cytel & ARCS Collaborate to Strengthen Early Phase Capabilities
A combination of industry and policy forces have recently changed the shape of Australia’s R&D sector, making it a...
Read article

May 3, 2022
Adaptive Designs for Early Phase Development: Are the questions right?
In 2005, Pfizer launched a Phase 1 trial for the kinase inhibitor crizotinib. Six years later, it was approved, thanks...
Read article

April 22, 2022
Cytel’s Single Arm Trials Panel Selected as a Part of ISPOR 2022
This past decade has undoubtedly witnessed an increase in the number of single arm trials submitted to HTA bodies....
Read article

April 8, 2022
Robust Trial Design Under Treatment and Enrollment Uncertainty
The planning and optimization of a clinical trial is beset by uncertainties: knowledge of treatment effects, the...
Read article

March 8, 2022
How to conduct better time-to-event analysis with delayed treatment effects
The issue of delayed treatment effects in immuno-oncology was demonstrated during a FDA-Industry sponsored workshop...
Read article

February 22, 2022
How and Why to Implement Optimal Adaptive Promising Zone Designs
When determining the best possible statistical design for a particular trial, large pharmaceuticals and small biotechs...
Read article

February 15, 2022
Measuring Estimates and Confidence Intervals in Adaptive settings?
As the use of advanced and innovative clinical trial designs continue to rise, sponsors often wonder which estimation...
Read article

February 2, 2022
How to Overcome Common Challenges to Patient Recruitment Projections
For nearly ten years, suboptimal trial enrollment has been cited as a primary cause of clinical trial discontinuation....
Read article

January 27, 2022
Winners of The Promising Zone Quiz
As a part of Cytel’s 10 Year Anniversary of the Promising Zone Design, Cytel hosted a quiz on “Keeping the Promise” –...
Read article

November 15, 2021
Optimal Promising Zone Designs: What Biotechs Need to Know
Since its first publication ten years ago, Cyrus Mehta and Stuart Pocock’s Promising Zone Design for sample size...
Read article

November 8, 2021
Keeping the Promise: Ten Year Anniversary of the Promising Zone Design
Ten years ago Cytel co-founder Professor Cyrus Mehta and Professor Stuart Pocock of the London School of Hygiene and...
Read article

October 21, 2021
Measuring Non-Adherence and Non-Persistence
A number of methods currently exist to measure non-adherence and non-persistence of medical therapies, for improved...
Read article

October 14, 2021
Weighting and Prioritization for Trial Selection: New Webinar
When choosing the optimal clinical trial design for a given study, sponsors face critical questions like choice of...
Read article

October 7, 2021
Non-adherence and Non-persistence
‘Drugs do not work in patients who do not take them,’ said former surgeon general C. Everett Koop. Unfortunately, the...
Read article

September 21, 2021
Three Underappreciated Benefits of Pareto for Empowered Trial Selection
Earlier this summer, we published a series of articles on the need to utilize weighting and prioritization tools in the...
Read article

September 8, 2021
Reconciling an old debate with modern technology
A key decision in the design of clinical trials in oncology involves whether to select progression free survival (PFS)...
Read article

August 18, 2021
What does reducing the risk of a faulty conclusion mean: Case study
During the design of a clinical trial, many biotechs want to substantially reduce the risk of a good new therapy being...
Read article

August 10, 2021
CLRPerm: Cytel Scientists and Collaborators Propose a New Method for Meta-analysis for Rare Events
A few weeks ago I wrote about new research conducted by Cytel statisticians, on the challenge of conducting...
Read article

August 9, 2021
When to make decisions? Strategic planning of interim looks.
Recently we discussed examining clinical development through a Bayesian lens, in honor of Cytel co-founder Nitin Patel...
Read article

July 28, 2021
Lessons Learned from Leveraging Computing Power for Clinical Strategy
“We found an optimal design in hours that might have taken months to find using standard methods,” reflected Fabien...
Read article

July 27, 2021
Thinking of Clinical Development from a Bayesian Lens
Program and portfolio optimization creates a framework throughout the course of the clinical development journey, that...
Read article

July 22, 2021
The Risk of Under-exploring Trial Design Options: A New Case Study
Earlier this year, Cytel founder Cyrus Mehta observed that clinical trial design is often treated like an art rather...
Read article

July 14, 2021
Novel Uses of Scoring Functions in Clinical Trial Design Selection
For decades, statisticians have cultivated methods to optimize and de-risk clinical trials for strong regulatory...
Read article

July 12, 2021
Using Confidence Distributions to Manage Statistical Heterogeneity
Medical researchers and public health experts are becoming more reliant on meta-analyses to capture in summary form,...
Read article

July 7, 2021
Ensuring You Get Optimal Study Power for Your Investment
Suppose a statistician were to tell a clinical trial sponsor that it was possible to improve the power of the sponsor’s...
Read article

June 30, 2021
Mathematical Methods for Clinical Trial Financial Strategy
When Cyrus Mehta introduced the Promising Zone Design over a decade ago, the new statistical method not only...
Read article

June 23, 2021
Eliminating Underperforming Clinical Trial Designs
Much of the discussion about clinical trial design considers methods to optimize performance characteristics and...
Read article

June 18, 2021
Use of Scoring Functions for Clinical Trial Optimization
Next week’s PSI Conference will feature Dr. Yannis Jemiai speaking on the use of Scoring Functions in the re-imagined...
Read article

June 15, 2021
The Promising Zone Ten Years Later
Ten years ago, a seminal paper published by Cytel Founder Cyrus Mehta, introduced the Promising Zone Design to...
Read article

June 9, 2021
Selecting a Clinical Trial Design: How Broadly Should You Explore?
When selecting clinical trial designs, how many design options should a sponsor explore? Would a sponsor feel more...
Read article

June 2, 2021
A Data-Infused Approach to De-Risking Clinical Trials
For many decades the Pareto Frontier has been employed by actors in the private sector to evaluate and understand the...
Read article
May 20, 2021
Quantitative Bias Analysis to Strengthen Comparative Effectiveness
As more payers and HTA agencies turn to real world data to compare the effectiveness of various treatment effects, two...
Read article

May 19, 2021
A Non-Technical Guide to Statistically-Informed Clinical Strategy
Clinical trial sponsors are more likely than ever to use the power of simulation and forecasting to evaluate the...
Read article

May 13, 2021
An Interview with Radek Wasiak, Head of Real World and Advanced Analytics at Cytel
Cytel’s HEOR and RWE Expertise has grown quite significantly in the past year. Could you speak a little bit about the...
Read article

May 12, 2021
Strategic Insights from Novel Bayesian Methods – Complimentary Paper
Did you know that Bayesian methods can strengthen Frequentist trials through the use of Bayesian decision criteria or...
Read article
May 4, 2021
New Publication on Adaptive Platform Trials
As healthcare systems across the world, continue to grapple with the pressures of COVID-19, Cytel advances yet another...
Read article

April 23, 2021
Bayesian Methods & Vaccines Research: COVID-19
The urgent need to discover and assess the efficacy and safety of COVID-19 vaccine candidates will affect the future...
Read article
April 2, 2021
Using Bayesian Networks to Predict Survival Outcomes: New Case Study
Earlier this month, my colleagues at Cytel Canada published a paper in JCO Clinical Cancer Informatics, offering a...
Read article
November 5, 2015
The 24 Hour Work Day
Oftentimes people perceive a tradeoff between speed and quality. The faster you do something the more likely you are to...
Read article
November 3, 2015
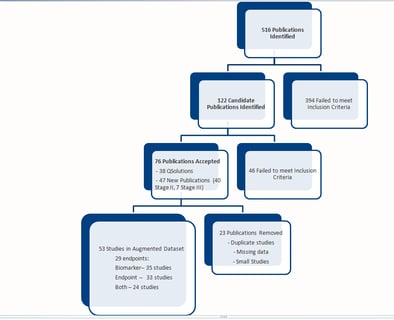
Pharmacometrics for Biomarker Driven Clinical Strategy
QPP (sometimes called QP2) remains at the heart of model based drug development. Short for Quantitative Pharmacology &...
Read article
October 27, 2015
P-Values & Pharma Development: We Want to Hear from You
Here at Cytel we have enjoyed following the debates on the p-value controversy currently taking place on the ASA...
Read article
October 19, 2015
3 Statistical Challenges for Pooling Phase 1 Data
It is often necessary to pool safety data from late phase studies, in preparation for regulatory submission. Some of...
Read article
October 1, 2015
It’s Time to Bridge the Gap Between Pharmacometrics and Biostats
This week marks the sixth annual American Conference on Pharmacometrics, held this year in Crystal City, VA. Situated...
Read article
September 24, 2015
Virtual Teams and Clinical Data Management
Earlier this week, Patti Arsenault, Cytel’s Global Head of Clinical Data Management, sat on an SCDM panel with members...
Read article
September 17, 2015
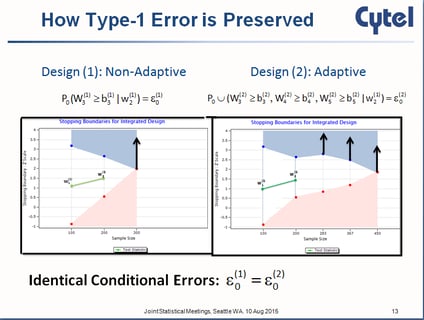
Inference on Confidence Intervals for Adaptive Designs: The Latest Breed of Adaptive Clinical Trials
Most people familiar with adaptive clinical trial designs are familiar with those statistical designs that reject the...
Read article

August 13, 2015
Do you really need a full service CRO? An exploration of strategic options
Full service or specialized? Full service or specialized? For many looking to hire a CRO, the answer is obvious....
Read article
August 10, 2015
Mitigate Phase 3 Clinical Trial Risk by Optimizing Phase 2 Data
When approaching a Phase 3 clinical trial, the need to ‘de-risk’ the massive investment often leads sponsors on a quest...
Read article
July 27, 2015
Evidence Based Medicine: 25 Years Later
We were saddened to learn earlier this year, of the passing of Professor David Sackett. Widely recognized as the father...
Read article

July 21, 2015
MCP-Mod for the Modern Dose-Ranging Clinical Trial
MCP-Mod methodology for dose-ranging clinical trials has been gaining popularity since the 2013 publication of the...
Read article
June 26, 2015
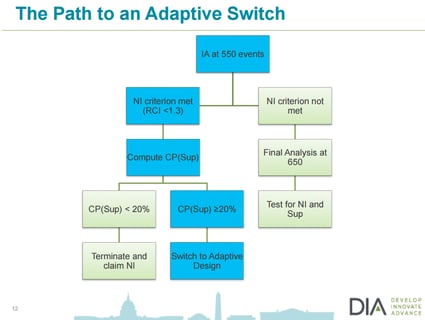
Clinical Trials: Why You Should Not Power for Superiority Upfront
Powering a trial for superiority can be financially risky. In some instances it may also prove unnecessary.
Read article
June 18, 2015
A Cautionary Tale about Composite Endpoint Construction: The ARISE Trial
In August 2006 AstraZeneca completed the ARISE trial, which aimed to determine whether AGI-1067 was effective in...
Read article
June 11, 2015
Aligning Clinical Development & Regulatory Objectives for Cardiovascular Outcome Trials
When the FDA first began to require pharmaceuticals to perform cardiovascular outcome trials to establish the safety of...
Read article
June 5, 2015
Building Teams to Handle Unexpected Regulatory Agency Requests
Not long ago, one of our clients submitted Phase 2 and Phase 3 data for a new rare disease drug which had received...
Read article

May 28, 2015
How to Use Outsourcing to Reduce Clinical Development Risk
Risks in drug development range from taking the wrong drugs forward to Phase 3 to investing in a drug development...
Read article

May 20, 2015
Seamless Adaptive Clinical Trials: What’s really at stake?
Seamless adaptive clinical trials have gained popularity for reducing the projected time it takes to complete the...
Read article

May 15, 2015
Can You Reproduce Your Clinical Trial Results?
Imagine that it’s been three years since the completion of a trial, and that suddenly a regulatory body calls into...
Read article

May 14, 2015
Marvin Zelen's 8 Predictions for the Future of Biostatistical Sciences
Ten years ago, in May 2005, world-renowned biostatistician Marvin Zelen was asked to deliver a keynote address before...
Read article

April 30, 2015
New Articles on Adaptive Clinical Trials & Adaptive Financing
Adaptive financing (not to be confused with adaptive licensing) explores how biotechs, pharmaceuticals and potential...
Read article
April 23, 2015
Dose-finding with Sequential Parallel Comparison Designs
Last week the Cytel Blog discussed the benefits of using the Adaptive Maximizing Design [AM Design] for dose-finding...
Read article
April 21, 2015
Adaptive SSR for Small Sample Sizes?
“We shouldn’t use an adaptive design, our sample size is too small.” Most clinical trial planners have heard this line...
Read article
April 16, 2015
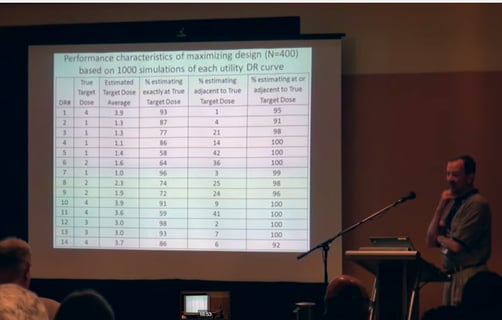
Phase 2 Designs for Clinical Utility Limiting Therapies
When testing certain types of new drugs it is known in advance that the adverse side-effects of the medication will...
Read article
April 9, 2015
Relative Clinical Efficiency and Phase 2 Biomarker Studies
Last year. Nature Reviews Drug Discovery asked the FDA’s Tatiana Prowell (Hematology & Oncology Products Division)...
Read article
April 2, 2015
Monte Carlo Simulations II: Reassessing Strategic Options During an Interim Look
Midway through a trial is a terrible time to realize that you need a new strategy to complete the study. Sadly, it is...
Read article
March 27, 2015
Leveraging the Flexibility of an Adaptive Clinical Trials: A Case Study
We have often said that one of the greatest benefits of an adaptive clinical trial is the flexibility it affords for...
Read article
March 26, 2015
Statistical and Operational Challenges of the VALOR Trial
Last year Sunesis completed the VALOR trial, the first clinical study to make use of the groundbreaking promising zone...
Read article
March 20, 2015
How Bayesian Strategies Can Expedite a Pediatric Clincial Trial Time by 20 - 40%
Sometimes a new candidate drug for a pediatric study has already been tested on adults for safety and efficacy. We know...
Read article
March 19, 2015
How to Plan Interim Looks in Adaptive Clinical Trials: 3 Strategies
A well-timed interim analysis can generally supply added benefits to the operational and administrative aspects of a...
Read article
March 12, 2015
Monte Carlo Simulations for Patient Enrollment: A Presentation
Recently, we published an interview with Chris Conklin, the Director of the Center for Feasibility Excellence at...
Read article
March 10, 2015
Embracing the Adaptive Mindset
Most of us are primed to think about the design of adaptive clinical trials as a narrow set of techniques applied to a...
Read article
March 5, 2015
Adaptive vs. Group Sequential Designs in Survival Analysis
The Mehta-Pocock promising zone is often used to carry out unblinded sample size re-estimation during interim analysis....
Read article
February 26, 2015
Successful Adaptive Confirmatory Dose-Response Pediatric Study
Cytel President and co-founder Cyrus Mehta has co-authored a paper on Infantile Hemangioma, recently published in the...
Read article
February 17, 2015
How Proposed Regulatory Reforms Will Affect Your Clinical Trial
21st Century Cures (also called Cures2015) is a bipartisan initiative undertaken by the Committee on Energy and...
Read article
February 10, 2015
How to Shorten a Cardiovascular Outcome Trial By Two Years
Cardiovascular outcome trials (CVOTs) have earned the reputation of being the untamable behemoths of the clinical...
Read article
February 5, 2015
3 Key Trends in Clinical Trial Enrollment Forecasting
Every clinical trial requires some manner of trial forecasting, normally for feasibility and patient enrollment....
Read article
January 29, 2015
Adaptive Design and Bayesian Statistics: 5 Years Later (Podcast)
February 2015 marks the five year anniversary of the FDA’s Guidance on Adaptive Design Clinical Trials for Drug and...
Read article
January 27, 2015
How to Take Control of Your Enrollment Woes
Last week we released an infographic on why Phase 3 trials fail. The numbers, while eye-opening, did not capture a...
Read article
January 22, 2015
Adaptive Clinical Trial Strategies for the Limited Early Phase Budget
The Journal of the American Medical Association recently published an article entitled ‘The Anatomy of Medical...
Read article
January 20, 2015
Why Drugs Fail in Phase 3: A Cytel Infographic
According to a recent Cytel Whitepaper on Adaptive Clinical Trials, 50% of Phase 3 trials eventually fail. This new...
Read article
January 16, 2015
Old and New Drug Development Paradigms: Cytel Infographic
Adaptive designs are thought to be the new paradigm in drug development, allowing statisticians and trial designers to...
Read article
January 13, 2015
Simulation and Prediction for Adaptive Licensing Decision-Making
Janus was the Roman God of transitions, a deity with two faces, one looking towards the past and the other the future....
Read article
December 18, 2014
Early Phase Development Strategy: Bayesian Methods for Go/No-Go Rules
Earlier this week, we at Cytel enjoyed a riveting in-house discussion on the uses of Bayesian decision rules for...
Read article
December 16, 2014
Cultivating Versatility in Statistical Consultants
Richard Branson once wrote: “I have always valued capability over expertise. While you may need to hire specialists for...
Read article
December 11, 2014
Adaptive Designs for Infectious Diseases Clinical Development Strategy
A common framework for the clinical development of vaccines involves the study of several candidate compounds in Phase...
Read article
December 9, 2014
Drug Supply Planning for Dose-Ranging Adaptive Trials
When planning a conventional trial, one can anticipate the drug supply necessary for the trial by determining how the...
Read article
December 4, 2014
Operationally Seamless & Inferentially Seamless Adaptive Designs
Fulyzaq® from Napo/Salix was the first drug ever to be approved using an adaptive two-stage "seamless" clinical trial...
Read article
November 18, 2014
A Bayesian Industry Approach to Phase I Combination Trials in Oncology
Statisticians and scientists at Novartis have been at the forefront of developing a new method in early phase oncology...
Read article
November 12, 2014
Ranking Adaptive Dose-Finding Designs using Clinical Utility Functions
Clinical utility functions provide Phase 2 trial sponsors with an intuitive metric by which to measure the quality of a...
Read article
November 6, 2014
Translational Statistics: How to Move Beyond the Comfort Zone
Professor LJ Wei holds that rules are for lawyers, not (necessarily) clinicians. When designing modern clinical trials,...
Read article
November 4, 2014
New Exploratory Trial Method Translates into Better Financial Strategy
A key stage of exploratory drug development is implementing a proof-of-concept study to demonstrate the safety of a...
Read article
October 28, 2014
Adaptive Dose Finding Using Toxicity Probability Intervals
Phase 1 oncology trials typically use either rule-based methods or model-based methods to determine the most acceptable...
Read article

October 21, 2014
Clinical Impact Beyond 'Time to First' Analyses
Every year, the East Users Group Meeting brings together notable experts from industry and academia to discuss the...
Read article
September 25, 2014
Part II: The Philosophy Behind a Software Package
A few weeks ago, we posted a synopsis of an event held at ISCB Vienna in which statisticians from Cytel, SAS and Stata...
Read article
September 18, 2014
5 times ‘Keep it Simple’ May Be Bad Advice for Clinical Designers
When designing clinical trials, many trial designers are advised to keep the trial simple. Prima facie, the keep it...
Read article
September 11, 2014
Empirical Study Confirms Positive Impact of Adaptive Designs
According to a recent Impact Report from the Tufts Center for the Study of Drug Development, 21% of active clinical...
Read article
September 4, 2014
Adaptive Designs for Evidence Based Oncology: Insights from the Experts
Imagine if we were to count the number of possible reasons that investigators might have for monitoring a biomarker...
Read article
September 2, 2014
Impact of Study Design and Development Strategy on Pharmaceutical Programs and Portfolios
As more clinical trials make use of adaptive designs, investors have come to realize that high quality trial designs...
Read article

August 26, 2014
Statisticians from Cytel, SAS and Stata talk Software Development
During an invited speakers session at the lnternational Society for Clinical Biostatistics, Cytel VP Yannis Jemiai was...
Read article
August 14, 2014
Backward Image Confidence Intervals for Adaptive Group Sequential Designs (Full Article Attached)
Cytel statisticians are looking foward to attending the Conference of the International Society for Clinical...
Read article

August 12, 2014
What Horsepower Can Teach us about Well-Powered Trials
Beyond Wild Horses: Developing Innovation at Cytel "Horse-and-pony" by arjecahn on flickr. -...
Read article
August 5, 2014
Reflections on Statistical Entrepreneurship: An Interview with Nitin Patel
Cytel CTO Nitin Patel, recently sat down with ECHOES (a magazine for statistics in clinical trials) to discuss his...
Read article
July 31, 2014
Bayesian Approaches in Clinical Trials: Updates on Tools & Techniques
Statisticians at Cytel are staunch advocates of the use of Bayesian methods in clinical trials. This summer's Joint...
Read article

July 29, 2014
Why Using Adaptive Designs Can Attract Investors to Your Trial
Adaptive designs are the unsurprising hot topic of this year’s Joint Statistical Meeting, which features over one...
Read article

July 24, 2014
Data Management and Biostatistics III: Statistical Innovation in Clinical Data Management
This is the third post in a three part series in which we consider (i) improvements to trial quality that result from...
Read article

July 22, 2014
10 Simple Steps to Deciding Whether Your Next Trial Should be Adaptive
PROCYSBI, the first drug to receive FDA approval after following an adaptive population re-assessment design, was one...
Read article
July 17, 2014
Predicted Interval Plots: A General Overview
In anticipation of Cyrus Mehta’s webinar next week on new predictive analytics tools for trial forecasting, we thought...
Read article
July 10, 2014
Designs for Biomarker Driven Population Enrichment in Oncology
Complexities with identifying suitable test populations in oncology studies contribute significantly to the 60%...
Read article
July 8, 2014
5 Reasons to Invest in Adaptive Designs for Population Enrichment
The above graphic is from Cyrus Mehta's slides on 'Adaptive Population Enrichment for Oncology Trials with Time to...
Read article
July 1, 2014
'Multivariate Approaches for Risk-Based Monitoring' An Adaptive Design (Slides Attached)
A recent Cytel Seminar on Adaptive Statistical Designs featured a talk by Michael Elashoff (Patient Profiles) on...
Read article
June 26, 2014
Adaptive Designs for Precision Medicine: A Look at Pfizer
The rise of biomarker based treatments in oncology has meant a reconceptualization of what constitutes a particular...
Read article
June 10, 2014
Cytel Weighs in on Strategies for Oncology Development
The FDA’s Tatiana Prowell (Breast Cancer Scientific Lead in the Office of Hematology & Oncology Products) recently gave...
Read article

June 3, 2014
Data Management & Biostatistics II: Operational Benefits of Bundling
This is the second post in a three part series in which we consider (i) improvements to trial quality that result from...
Read article
May 29, 2014
2014 Zelen Award Honors Statistician and Educator
Cytel has taken the initiative to train the next generation of clinical programmers through its innovative Clinnical...
Read article

May 22, 2014
Data Management & Biostatistics I: Improving Trial Quality
This is the first of a three part post in which we will consider (i) improvements to trial quality that result from...
Read article
May 8, 2014
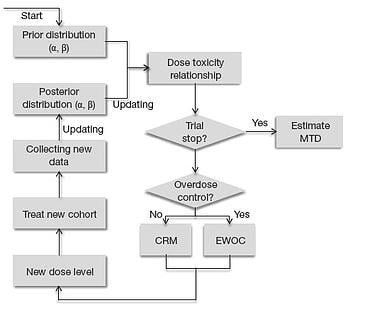
5 Reasons to Invest in Bayesian Dose-Escalation Methods
( Editor's note: This post has been refreshed in December 2016) Model based algorithms for Phase I dose-escalation have...
Read article
May 5, 2014
The Perils of Poor Recruitment
A new JAMA study on discontinued randomized trials in Switzerland, Germany and Canada, reports that poor recruitment...
Read article

April 21, 2014
StatXact 25th Anniversary: A Horizon for the Stars
The core methodological problem that would eventually spur the development of Cytel’s StatXact software was first posed...
Read article


