Weighting and Prioritization for Trial Selection: New Webinar

When choosing the optimal clinical trial design for a given study, sponsors face critical questions like choice of primary endpoint or specification of target population. Resource limitations, in the form of patients or even financing might further reduce viable options. Still, a strategic life sciences leader likely has a wealth of other questions specific to the given context of a study. How should these questions be addressed quickly across multiple stakeholders, for empowered trial selection?
These questions might include questions like: What happens if there is a delayed treatment effect of one month? When trying to maintain power at 80% what are the tradeoffs in speed and cost? Computing answers for these questions requires a combination of sophisticated algorithms, and millions of simulations that enable statisticians to see patterns emerging between different performance characteristics of a clinical trial.
A recent webinar by Cytel’s Chief Scientific Officer, Dr. Yannis Jemiai, walks through the decision-making on a carcinoma study, where sponsors had these and numerous other questions relevant for decision-making.
According to Dr. Jemiai, sponsors facing such questions currently depend on an ad hoc decision-making process, where modeling, risk analysis, cost and stakeholder opinion are combined in no particular order. This leads to confusion about what to include and exclude in the decision-making process, how to achieve consensus when opinions differ, and so forth. Dr. Jemiai advocates for an Evolving Decision-Making Process that streamlines inputs like modeling and risk analysis into structured decision-making.
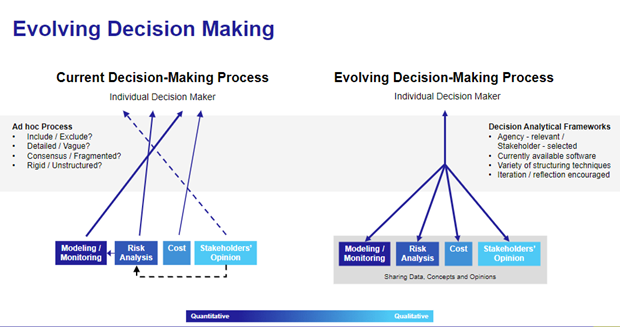
Dr. Jemiai then takes the audience through three elements of such decision-making. First he highlights the importance of eNPV maximized designs, which “focus the conversation on value.”
Such designs offer an intuitive way to tie measurements of ROI directly back to study design. He then explains the use of multi-criteria decision-analysis (MCDA) that creates transparency when multiple stakeholders are handling multiple (often contradictory) criteria. Finally, he reviews designs that are Pareto Optimized, which ensure that sponsors are not leaving valuable opportunities on the table, simply because there has not been sufficient time to evaluate sufficient study design options.
Altogether, Dr. Jemiai presents a new decision-making framework that combines enhanced weighting and prioritization tools with trusted, intuitive technology. Click below to learn more:
About the Author of Blog:

Dr. Esha Senchaudhuri is a research and communications specialist committed to helping scholars and scientists translate their research findings to public and private sector audiences. She received a doctorate from the London School of Economics in philosophy, and is a former early-career fellow at the American Academy of Arts and Sciences. She has taught medical ethics at the Harvard School of Public Health (TH Chan School) and sits on the Steering Committee of the Society of Women in Philosophy's Eastern Division, which is responsible for awarding the Distinguished Woman Philosopher Award.
h


