April 8, 2024
In this latest edition of the Career Perspectives series, we are excited to introduce our readers to Joshua Murray,...
Read article
March 4, 2024
In this new installment of the Career Perspectives series, we had the privilege of interviewing Arnold van Aswegen,...
Read article
June 9, 2023
In the ever-evolving landscape of clinical development, the need for robust evaluation of interim clinical data through...
Read article
June 7, 2023
Since its founding in 1987, Cytel has been home to some of the most passionate, creative, and talented experts in the...
Read article
January 25, 2023
People think in Bayesian terms all the time: we use prior information and the evidence at hand to make decisions in our...
Read article
December 6, 2022
In this edition of the Career Perspectives series, I spoke with Veronica Chan, Principal Clinical Data Manager at...
Read article
November 9, 2022
In this edition of the Career Perspectives series, I interview Malte Stein, Senior Biostatistician. Malte owned the...
Read article
October 14, 2022
In this edition of the Career Perspectives series, I interview Nicolas Rouillé, Senior Director, Statistical...
Read article
August 4, 2022
In this edition of the Career Perspectives series, I interview Allison Luccock, Director of Business Operations for...
Read article
June 13, 2022
The International COVID-19 Data Alliance (ICODA) was formed to address the challenge of generating rapid and rigorous...
Read article
May 24, 2022
Traditionally, clinical trials are expensive, long in duration, and have low success rate. But with the advent of rich...
Read article
May 12, 2022
Synthetic control arm (SCA) methods are statistical methods that are seeing rapidly increasing use in comparative...
Read article
May 5, 2022
While randomized control trials remain the industry gold-standard for regulatory and reimbursement submissions, there...
Read article
May 4, 2022
If you are a pharmaceutical or biotech company seeking to enter the market with a new drug, you need to submit a...
Read article
April 29, 2022
At ISPOR US 2022, Cytel’s HEOR & RWE experts will be contributing to a range of Issue Panels, In-person Podium...
Read article
April 21, 2022
Sophisticated Bayesian Methods are gaining a lot of traction as they bring flexibility and speed to clinical trial...
Read article
April 13, 2022
The Life Sciences landscape has seen an impactful digital evolution in the past two years. The pandemic has accelerated...
Read article
April 12, 2022
In this edition of the Career Perspectives series, I interview Charles Warne, Associate Director of Biostatistics at...
Read article
February 9, 2022
Data is the cornerstone of any clinical trial and is used to ultimately drive the decision-making process related to...
Read article
January 26, 2022
In this edition of the Career Perspectives series, I interview Jessica Bhoyroo, Cytel Clinical Data Manager based in...
Read article
January 20, 2022
Limited patient populations resulting in small study sample sizes is a difficulty associated with the development of...
Read article
December 20, 2021
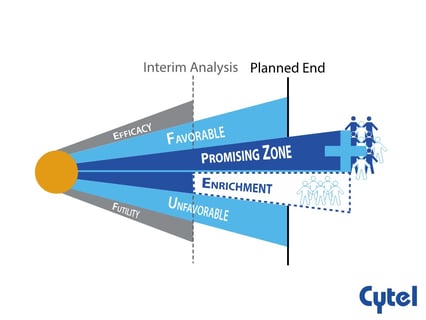
The promising zone design is an adaptive design which allows for sample size re-estimation based on the results of an...
Read article
December 8, 2021
Despite the debate in the scientific community on adaptive sample size reassessment (SSR), noteworthy developments have...
Read article
December 7, 2021
Sponsors bringing a successful new product to market have to overcome two hurdles: submission and reimbursement. For...
Read article
November 30, 2021
The aim of any clinical research is to detect the actual difference in treatment effect between two groups (power) and...
Read article
November 23, 2021
A randomized clinical trial (RCT) is the gold standard approach to demonstrate the efficacy and safety of novel...
Read article
November 17, 2021
Traditionally, the teams responsible for clinical development and regulatory submissions do not consult the market...
Read article
November 11, 2021
The value of Real World Evidence (RWE) is well known to many stakeholders, but its full potential for market access and...
Read article
November 10, 2021
There are many reasons why traditional approaches to designing a clinical study are generally suboptimal and do not...
Read article
November 4, 2021
The prominent European conference for Health Economics and Outcomes Research (HEOR), ISPOR Europe 2021, is around the...
Read article
November 2, 2021
A good clinical study design performs well not only under the ideal target scenario. Statisticians should be able to...
Read article
October 27, 2021
A staged investment strategy aligns R&D decisions and financial planning with the interim looks of a clinical trial. If...
Read article
October 22, 2021
Currently, there are many treatment options for Cancer such as, Immunotherapy, Radiation Therapy, Chemotherapy etc. If...
Read article
October 20, 2021
For health innovators, trial selection is a key success factor as there are no second chances. But how do you find the...
Read article
October 6, 2021
Master Protocols are advanced and innovative clinical trial designs that can evaluate multiple therapies and disease...
Read article
October 1, 2021
The COVID-19 pandemic elevated the challenge of designing and executing clinical trials within a substantially...
Read article
September 24, 2021
We may be familiar with adaptive designs, but their complexity has made them difficult to implement and their benefits...
Read article
September 14, 2021
Alyssa Biller is a Senior Solutions Consultant at Cytel and currently plays a primary role in onboarding customers for...
Read article
August 20, 2021
In clinical research, decision makers need to choose among different courses of action every day. Whether it is...
Read article
August 13, 2021
With the cloud computing power that we have today, we can run simulation of 1000s of designs with each design...
Read article
August 5, 2021
It is important to compare competing interventions to determine value of medicines, both from clinical and societal...
Read article
July 21, 2021
Conducting a transparent, targeted and robust benefit-risk assessment of a new drug or product is one of the most...
Read article
July 20, 2021
Anil Golla is Vice President, Functional Service Provision (FSP) at Cytel. After 17 years of working at pharmaceutical...
Read article
July 1, 2021
Synthetic control arms (SCAs) are best suited for situations when a single arm trial is run in a patient population...
Read article
June 25, 2021
The main challenge associated with the development of therapies for rare diseases is typically the small study sample...
Read article
June 24, 2021
Synthetic control arms (SCAs) leverage real world data from various sources or evaluations of historical clinical data...
Read article
June 17, 2021
In Part 2 of this four-part blog series, we bust another myth surrounding synthetic control arms (SCAs) used in...
Read article
June 14, 2021
ACDM’s (Association for Clinical Data Management) eDigital data management expert group (DMEG) focuses on driving...
Read article
June 10, 2021
Over the past decade, single arm trials have emerged as an accepted way of assessing a new treatment intervention....
Read article
June 4, 2021
Bayesian methods have been playing a key role in transforming clinical research in therapeutic areas such as oncology...
Read article
June 3, 2021
Bayesian methods allow for the incorporation of prior knowledge, in terms of either expert opinion from clinicians or...
Read article
May 7, 2021
When is a study design considered to be optimal? A good design performs well not only under the ideal target scenario...
Read article
May 5, 2021
Cytel’s team of HEOR and Real-World Evidence (RWE) experts are all set to make a splash at the Virtual ISPOR on May 17...
Read article
April 21, 2021
Two therapies are placed in head-to-head clinical trials when they are compared against each other as opposed to a...
Read article
April 14, 2021
In the era of modern clinical development, several companies are turning towards novel and innovative approaches such...
Read article
April 13, 2021
There has been an increasing use of digital measures in drug development recently. New wearables technologies can help...
Read article
April 7, 2021
Scientists at Cytel recently published a paper in the Journal of the American Medical Association (JAMA). Among the...
Read article
September 12, 2019
"If you went to bed last night as an industrial company, you're going to wake up today as a software and analytics...
Read article