Dr. Julia Edwards Findings Promising Zone Designs
The aim of any clinical research is to detect the actual difference in treatment effect between two groups (power) and to provide an estimate of the difference with a reasonable accuracy (precision). [1] Sample size calculation, performed during the planning and designing stage of the study, require assumptions regarding treatment response and variability. This often leads to under or over-powered trials when prior information is limited.
Adaptive sample size re-estimation methods allow sample size to be adjusted at an interim analysis. Such designs allow for one interim look that determines whether a trial ought to be stopped for futility, stopped for efficacy, continued as planned, or continued with a larger sample size. Unblinded sample size re-estimation designs use accruing trial data to reassess sample size calculation assumptions and modify sample size if necessary. Some methods use conditional power to make interim decisions.
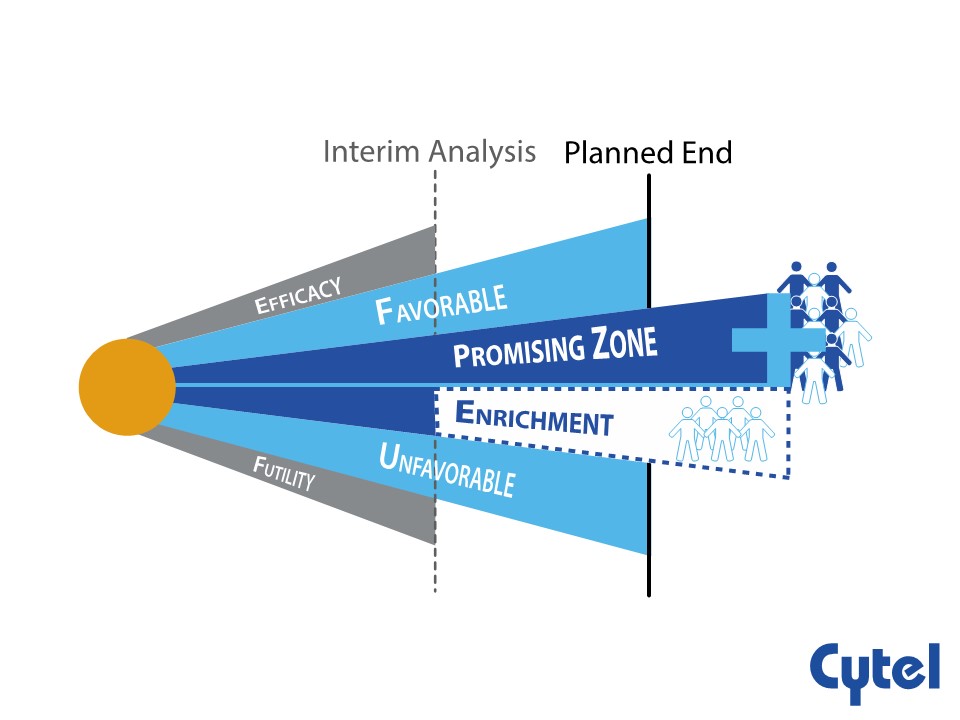
One such design is the promising zone design, a special form of sample size re-estimation whereby a study falls into one of five zones during interim analyses. The efficacy and futility zones are familiar from most group sequential designs. Additionally, there are Unfavorable and Promising Zones, which determine how well a therapy or intervention is performing.
Cytel is celebrating 10 Years of the Promising Zone Design with a series of webinars and panel discussions on the past and future of this groundbreaking design. In our latest webinar, Dr. Julia Edwards, Statistician at the Intensive Care National Audit and Research Centre (ICNARC) in London, presents a systematic review of the promising zone literature and a retrospective re-analysis of published trial data.
‘A systematic review of the “promising zone” design’ published in 2020, [2] provides an overview of the literature published on the promising zone, since its inception in 2011. This research comprehensively identifies the reporting of methodological research and provides details of trials implementing the design in practice.
During the webinar, Dr. Edwards offers insights on how the promising zone design is easy to implement and interpret and reduces risk of an underpowered trial. They are seen to be useful for small companies as they allow committing resources in stages. The review also reveals how promising zone designs enable time, cost and sample size savings in seamless Phase 2/3 studies. One area of concern identified is the use of the observed current trend assumption in the conditional power equation, effectively using the observed interim treatment effect twice and therefore potentially yielding an unstable estimate.
Dr. Edwards presents the results from a retrospective re-analysis of real-world clinical trial data, investigating alternative conditional power assumptions of the future treatment effect, and the impact on interim analysis decisions for unblinded sample size re-estimation designs. She also explores the stability of the treatment effect and makes recommendations on the timing of an interim analysis.
Learn more by clicking the link below:
Reference:
[1] Das S, Mitra K, Mandal M. Sample size calculation: Basic principles. Indian J Anaesth. 2016;60(9):652-656. doi:10.4103/0019-5049.190621
About the Author of Blog:
 Mansha Sachdev specializes in content creation and knowledge management. She holds an MBA degree and has 11 years of experience in handling various facets of marketing, across industries. At Cytel, Mansha is a Content Marketing Manager and is responsible for producing informative content that is related to the pharmaceutical and medical devices industries.
Mansha Sachdev specializes in content creation and knowledge management. She holds an MBA degree and has 11 years of experience in handling various facets of marketing, across industries. At Cytel, Mansha is a Content Marketing Manager and is responsible for producing informative content that is related to the pharmaceutical and medical devices industries.


