
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

March 13, 2024
Group sequential clinical trial designs — a type of adaptive clinical trial design — have emerged as a powerful tool in...
Read article

March 11, 2024
Quantitative Strategies for Rare Disease Clinical Trials
In 2023, rare diseases accounted for 30% of product pipeline under development, about half of which comprising...
Read article

January 26, 2024
Parameter Optimization of Multi-Arm Multi-Stage Designs
Innovations in the process of designing adaptive clinical trials have unlocked new possibilities for designing and...
Read article
October 4, 2023
How to Ensure Your Adaptive Trial Is Appropriate for Regulatory Submission
Adaptive clinical trial designs have become increasingly popular among developers and investors due to the many...
Read article
September 29, 2023
Innovative Clinical Trial Design: Commercial vs Open-Source Software? Why Not Both?
In the ever-changing field of clinical trial design, there is often a need to evaluate design options quickly and...
Read article

September 22, 2023
Myth Busting: Master Protocol Edition
Interest and appetite for master protocols is growing as sponsors consider opportunities in various therapeutic areas...
Read article

March 13, 2023
Industry Voices: Yannis Jemiai on Simulation-Guided Design and the Changing Landscape of Clinical Trial Strategy
Many industries have long since adopted the practice of modeling and simulating experimental scenarios. And despite...
Read article

February 3, 2023
Focal Points and Monetization: New Uses of Pareto Frontiers in Clinical Development
For clinical development and research and development teams, the Pareto Frontier can perform two functions. Let’s take...
Read article

January 27, 2023
To Adapt or Not to Adapt? A Decision Framework
Should your clinical trial be adaptive? Trials that include a prospectively planned modification based on an interim...
Read article

January 4, 2023
A Look Ahead for 2023
Returning to Cytel after the winter holidays, I am excited to begin a year that will likely prove memorable for both my...
Read article

December 29, 2022
Top Perspectives Articles of 2022
Perspectives on Enquiry and Evidence explores a wide variety of topics within clinical trial design and data science in...
Read article

December 27, 2022
Top Bayesian Topics of 2022
Bayesian methods have been playing a key role in transforming clinical research, providing a variety of new...
Read article

December 21, 2022
Top Interviews of 2022: Industry Voices and Career Perspectives
Perspectives on Enquiry and Evidence features two recurring interview series: Our new Industry Voices series, in which...
Read article

December 19, 2022
Top Adaptive Clinical Trial Topics of 2022
Adaptive trial designs – that is, trials that include a prospectively planned modification based on an interim analysis...
Read article

December 16, 2022
Topics in Bayesian Statistical Methods
Bayesian methods have been playing a key role in transforming clinical research, and Bayesian topics are frequently...
Read article

December 2, 2022
Celebrating 35 Years of Innovation and Impact: An Interview Series
For 35 years, Cytel’s scientific rigor and operational excellence have enabled biotech and pharmaceutical companies to...
Read article

November 29, 2022
Bayesian Adaptive Clinical Trial Designs: INLA vs. MCMC
Bayesian methods have continuously played a key role in transforming clinical research in therapeutic areas such as...
Read article

October 24, 2022
Cyrus Mehta on the Founding of Cytel
On the occasion of Cytel’s 35th anniversary, co-founder Professor Cyrus Mehta sits down with Dr. Esha Senchaudhuri to...
Read article

October 18, 2022
Nitin Patel on 35 Years of Technological Innovation
On the occasion of Cytel’s 35th anniversary, co-founder Professor Nitin Patel sits down with Dr. Esha Senchaudhuri to...
Read article

October 11, 2022
Joshua Schultz on the Evolution of Cytel
On the occasion of Cytel’s 35th anniversary, our CEO Joshua Schultz sits down with Dr. Esha Senchaudhuri to discuss the...
Read article

October 5, 2022
Platform Trials, Can they Benefit Animal Studies?
Master protocols and platform clinical trials have become an innovative and efficient approach to testing multiple...
Read article

October 4, 2022
MCMC vs. INLA in Bayesian Adaptive Clinical Trial Designs
Integrated Nested Laplacian Approximations (or INLA) are now starting to be used by statisticians as a key tool for...
Read article


September 23, 2022
Adaptive Trials at the Mainstream of Drug Development
Adaptive trial designs – that is, trials that include a prospectively planned modification based on an interim analysis...
Read article

September 12, 2022
On Frequentist and Bayesian Sequential Clinical Trial Designs
In clinical trials, patient enrollment is often staggered, with data collected sequentially. When designing a clinical...
Read article

January 21, 2022
How to Use and Interpret the Results of a Platform Trial
For our first Winter Weekend Read, Cytel presents How to Use and Interpret the Results of a Platform Trial, a JAMA...
Read article

October 29, 2021
Designing Platform Trials
A platform trial, a type of Master Protocol, is an experimental infrastructure to evaluate multiple treatments and/or...
Read article

October 22, 2021
The Benefits of Using Basket Studies in Oncology
Currently, there are many treatment options for Cancer such as, Immunotherapy, Radiation Therapy, Chemotherapy etc. If...
Read article

October 15, 2021
Applications of Master Protocols in a Global Health Context
Almost 3000 registered trials were performed in COVID-19 and a majority of them have been small and likely...
Read article

October 6, 2021
The Rapidly Evolving Need for Master Protocols
Master Protocols are advanced and innovative clinical trial designs that can evaluate multiple therapies and disease...
Read article

October 1, 2021
Dr. Kyle Wathen talks about the Need for Statistical Innovation
The COVID-19 pandemic elevated the challenge of designing and executing clinical trials within a substantially...
Read article

September 29, 2021
How to leverage model-informed drug development for rare diseases
While there is a plethora of rare diseases, some 7000 diseases and counting, one needs to consider the statutory...
Read article

September 24, 2021
ICT Magazine Interviews Dr. Yannis Jemiai on the Benefits of Bayesian
We may be familiar with adaptive designs, but their complexity has made them difficult to implement and their benefits...
Read article

June 30, 2021
Mathematical Methods for Clinical Trial Financial Strategy
When Cyrus Mehta introduced the Promising Zone Design over a decade ago, the new statistical method not only...
Read article

June 23, 2021
Eliminating Underperforming Clinical Trial Designs
Much of the discussion about clinical trial design considers methods to optimize performance characteristics and...
Read article

June 18, 2021
Use of Scoring Functions for Clinical Trial Optimization
Next week’s PSI Conference will feature Dr. Yannis Jemiai speaking on the use of Scoring Functions in the re-imagined...
Read article

June 15, 2021
The Promising Zone Ten Years Later
Ten years ago, a seminal paper published by Cytel Founder Cyrus Mehta, introduced the Promising Zone Design to...
Read article

June 9, 2021
Selecting a Clinical Trial Design: How Broadly Should You Explore?
When selecting clinical trial designs, how many design options should a sponsor explore? Would a sponsor feel more...
Read article

June 2, 2021
A Data-Infused Approach to De-Risking Clinical Trials
For many decades the Pareto Frontier has been employed by actors in the private sector to evaluate and understand the...
Read article

May 14, 2021
Starting the Conversation Early: Incorporating Business Considerations in Optimal Selection of Trial Design
When developing clinical strategy, applying familiar business principles to the specific requirements of clinical...
Read article
May 4, 2021
New Publication on Adaptive Platform Trials
As healthcare systems across the world, continue to grapple with the pressures of COVID-19, Cytel advances yet another...
Read article

April 28, 2021
Trial Selection: From Art to Science
Recently, Cytel co-founder Professor Cyrus Mehta noted that, “Clinical trial design selection is too much like an art,...
Read article

April 23, 2021
Bayesian Methods & Vaccines Research: COVID-19
The urgent need to discover and assess the efficacy and safety of COVID-19 vaccine candidates will affect the future...
Read article

January 26, 2021
Cytel Thought Leadership on Power of Bayesian Methods
Bayesian models offer a flexible way of incorporating historical controls in the analysis of trial data (whether single...
Read article

December 17, 2020
2020 Recap by Yannis Jemiai, Chief Scientific Officer, Cytel
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article

December 16, 2020
2020 Recap by Pantelis Vlachos, Principal/Strategic Consultant, Cytel
As we prepare to close the door on 2020, we asked Pantelis Vlachos, Principal/Strategic Consultant for Cytel, to share...
Read article

December 15, 2020
Satisficing, Optimizing and Globally Optimizing Trial Designs
When designing clinical trials, biostatisticians and clinical development teams are often faced with a conundrum. Given...
Read article
December 9, 2020
7 Key Features of Strategic Clinical Trial Design
As a part of Cytel’s Advanced Design Framework, a new Framework for the statistical design of clinical trials, Cytel...
Read article
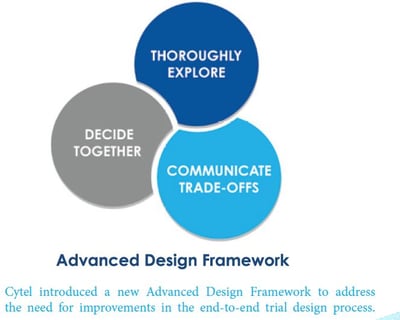
December 3, 2020
New Whitepaper: Reimagining Clinical-Trials
Increasing Clinical Development Productivity Using Statistics and Cloud-Computing The need for Re-imagining Clinical...
Read article

December 2, 2020
Program and Portfolio Optimization: A New Paradigm
Significant advances have been made to enhance the efficiency of clinical trial designs. However, the traditional...
Read article

November 18, 2020
We can design over 100,000 clinical trials in less than an hour
The current state of the clinical trials industry faces a challenge that was only hypothetical three or four years ago....
Read article
November 17, 2020
Role of Prediction and Causal Inference in Clinical Research
As a part of Cytel’s "New Horizons Webinar Series", Alind Gupta, Senior Data Scientist, presents case studies from his...
Read article

November 16, 2020
Bayesian Methods for Multiple Cohort Expansion (MuCE) designs
MUCE is a Bayesian solution for cohort expansion trials where multiple dose(s) and multiple indication(s) are tested in...
Read article

November 11, 2020
Interview with Yannis Jemiai: Advanced Design Framework
The widespread use of cloud-computing has altered the clinical trial design process. Whereas three or four years ago,...
Read article
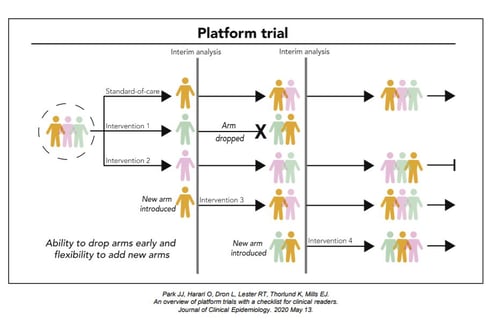
November 10, 2020
Key Design Considerations for Platform Trials
Platform trials are a new type of clinical trials where multiple interventions can be evaluated simultaneously against...
Read article

November 4, 2020
Cytel Introduces Advanced Design Framework: Part 3 - Communication Techniques to Ensure Alignment on Data-Driven Clinical Trial Designs
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought leaders that draws on...
Read article

October 29, 2020
Advanced Design Framework: Part 2 - A Quantitative Evaluation Approach
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought leaders that draws on...
Read article

October 21, 2020
Advanced Design Framework: Part 1 - Exploration of Design Space
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought-leaders after a decade...
Read article

October 15, 2020
An Advanced Design Framework for Clinical Development in the Era of Cloud-Computing
For over a decade, advanced trial design techniques have promised efficient trials with accelerated timelines,...
Read article

October 5, 2020
The Meta-Analytic-Predictive Priors Generation and Comparisons
Staying abreast of the rapid pace of clinical development means adopting innovative or computationally intensive...
Read article
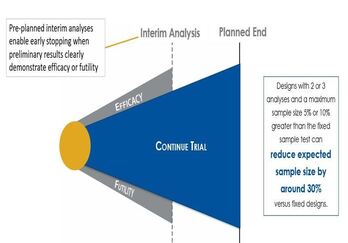
October 1, 2020
Improve Trial Design with Sequential Design and Sample Size
Methods involving Group Sequential Designs are one of the earliest deviations from a traditional two-arm clinical trial...
Read article

September 30, 2020
A Virtual Event Brought to you by Cytel and Novartis on Innovations
Today, there is a need for advanced quantitative techniques to combine all available information for better decision...
Read article

September 29, 2020
The New Horizons Series: Adaptive Multi-arm Multi-stage Clinical Trials
Innovation in trial designs are offering new routes forward for organizations of any size. They are now also aligned...
Read article

September 28, 2020
Advantages of platform designs for investigating COVID-19 therapies
Cytel has recently designed and implemented the TOGETHER Trials, funded by the Bill & Melinda Gates Foundation to...
Read article

September 9, 2020
Importance of Designing Clinical Trials from a Program Perspective
Cytel’s co-founder, Nitin Patel, conducted a webinar on designing clinical trials from a program-level perspective. His...
Read article

August 26, 2020
Career Perspectives: Interview with Yannis Jemiai, Chief Scientific Officer
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article

August 25, 2020
Nitin Patel on Designing Clinical Trials from a Program Perspective
It is important to take a strategic approach to clinical development in order to minimize the potential for Phase 3...
Read article

August 19, 2020
Webinar on Adaptive Designs for Dose Finding: Part 2
Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson provided...
Read article

August 13, 2020
Webinar: Adaptive Designs for Dose Finding
Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson provided...
Read article

August 12, 2020
Virtual Careers Open Day at Cytel
Cytel’s Biostatistics and Statistical Programming team provides integrated solutions, by blending the expertise of...
Read article

July 13, 2020
Interview with Dr. Thomas Burnett on Adaptive Enrichment
Cytel is hosting a complimentary webinar series that introduces biostatisticians and other members of the development...
Read article

July 7, 2020
Overcoming Clinical Development Challenges in Oncology with Innovative
Having its roots in the seminar rooms of the Dana Farber Cancer Institute, Cytel has a long record of establishing new...
Read article

June 24, 2020
Webinar - Practical Model-based Approaches for Phase I Oncology Trials
Last week, Cytel conducted its third webinar in the new introductory webinar series on Complex Innovative Trial...
Read article

June 15, 2020
Significance of Bayesian Model-Based Approaches in Oncology Trials: An Interview with Dr. Satrajit Roychoudhury
Cytel conducted a webinar with Dr. Satrajit Roychoudhury, Senior Director, Statistical Research and Data Science...
Read article
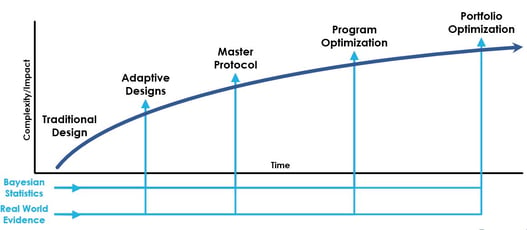
June 1, 2020
Webinar Replay: Innovative Drug Development at a Glance
In a recent interview with Cytel, Zoran Antonijevic, longstanding chair and leader of the DIA Adaptive Design...
Read article

May 28, 2020
Implications for the Future of Drug Development in Emerging Economies
On May 7, Cytel and Certara conducted a virtual panel discussion on new opportunities and implications for the future...
Read article

May 18, 2020
Interview with Zoran Antonijevic on Adaptive Design Methods
In this blog, we speak with Zoran Antonijevic, longstanding chair and leader of the DIA Adaptive Design Scientific...
Read article
April 29, 2020
Webinar: Transparent Machine Learning in Oncology
In our previous blog, we spoke with Alind Gupta, who works as a Machine Learning Researcher at Cytel in Canada. The...
Read article

April 20, 2020
Interview with Alind Gupta: Transparent Machine Learning in Oncology
Cytel is hosting a webinar on Transparent Machine Learning in Oncology, on April 21, 2020. Our speaker, Alind Gupta,...
Read article
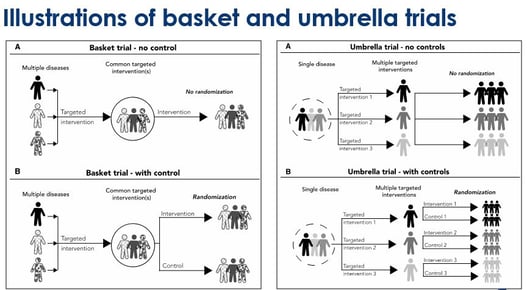
March 31, 2020
Key Design Thoughts for Basket Trials and Umbrella Trials by Jay Park
Since 1953, when the discovery of the structure of DNA was made, we have seen great advancements in genomics....
Read article

March 12, 2020
Interview with Jay Park: The present and future of Master Protocols
In September 2018, the FDA provided a draft guidance on master protocols reflecting an increased interest in these...
Read article

February 20, 2020
Unlock the power of your clinical data with these five top tips
It is widely acknowledged among drug developers that one of their most important assets is the data generated during...
Read article
January 30, 2020
Designing Event-based Studies: Interview with Pantelis Vlachos
The Cytel Trial Design Innovations (CTDI) Webinar Series recently hosted a webinar on designing event-based studies....
Read article

January 16, 2020
Adaptive Population Enrichment in a Phase III Oncology Trial
January’s Cytel Trial Design Innovations (CTDI) Webinar Series will feature Biostatistician and pioneering Bayesian...
Read article

January 8, 2020
How to optimize your data strategy to drive success in clinical development
In clinical development, data is the vital ‘foundation’ that supports your programs. To successfully bring a promising...
Read article

November 5, 2019
Drug Development in Rare Diseases - Innovation in Statistical Thinking
Cytel is delighted to have Kannan Natarajan speaking at the “Complex Innovative Trial Design Symposium and East User...
Read article

November 12, 2018
Can Statisticians Contribute to Enhance the Position of Patients in Clinical Trials?
In this blog, we talk with Robert Greene, Founder and President of the HungerNDThirst Foundation, about his upcoming...
Read article

October 23, 2018
Career Perspectives: Interview with Munshi Imran Hossain, Senior Data Scientist
Cytel data scientists apply advanced statistical techniques including predictive modeling of biological processes and...
Read article

October 5, 2018
Interview with Stephen Senn: 70 Years and Still Here: The Randomized Clinical Trial and its Critics
We are delighted that Stephen Senn will be joining us at the EUGM on November 14th and 15th in Darmstadt, Germany. In...
Read article

September 27, 2018
Decision Making in Development Programs with Targeted Therapies: with Heiko Götte
In this blog, we talk with Heiko Götte, Senior Expert Biostatistician at Merck about his upcoming presentation at...
Read article

September 20, 2018
Career Perspectives: Interview with Adam Hamm, Director of Biostatistics
At Cytel we believe that expert statistical input has the power to shape the future of clinical development: de-risking...
Read article

August 23, 2018
Career Perspectives: Interview with Meredith Alm, Manager, QA Compliance
Cytel has grown significantly over the last 30 years, with operations across North America, Europe, and India. All of...
Read article

August 15, 2018
2018 East User Group Meeting Addresses Multiplicity Themes, with keynotes including Stephen Senn and Meinhard Keiser.
Cytel’s 7th East User Group Meeting (EUGM) will take place on November 14 & 15, 2018 at Merck in Darmstadt, Germany,...
Read article

July 24, 2018
Career Perspectives: Interview with Sam Hsiao, Associate Director, Strategic Consulting
At Cytel our strategic consulting team works on a wide range of projects including: Identifying the best clinical trial...
Read article

July 3, 2018
Unveiling New East 6.5 Modules: Join Our Webinar
It’s shaping up to be a busy year for Cytel’s software development team with a number of upgrades and planned launches...
Read article

July 2, 2018
Highlights from the PSI 2018 Conference
A number of the Cytel team were in Amsterdam, 3rd- 6th June 2018 for the PSI Conference. This year’s conference was...
Read article

May 23, 2018
Addressing Critical Unmet Oncology Needs in the Era of Precision Medicine
Our Industry Voices series showcases our clients’ innovative work and breakthrough therapeutics in oncologic...
Read article

May 16, 2018
Rewriting the oncology textbook with cell-based immunotherapies
Our Industry Voices series showcases our clients’ innovative work and breakthrough therapeutics in oncologic...
Read article

May 9, 2018
Innovative Oncology Trial Designs in Practice
As we prepare to head to ASCO in under a month's time, we are pleased to share a new ebook that showcases some key...
Read article

February 28, 2018
Insight into the Coordination of Rare Diseases at Sanford registry
There is a consensus in the industry that data on rare diseases is limited, incomplete, and difficult to find or...
Read article

February 13, 2018
Career Perspectives: Interview with Ursula Garczarek, Associate Director - Strategic Consulting
Our strategic consulting team work on projects such as: Identifying the best clinical trial design, implementing...
Read article

January 26, 2018
6 Innovative Trial Design Videos
The Cytel YouTube Channel hosts a wealth of video presentations from Cytel experts as well as external industry and...
Read article

October 31, 2017
Webinar Replay: Dual Target Methods for Go/No-Go Decision Making
As part of Cytel's new Trial Innovations Webinar Series, Pat Mitchell, Statistical Science Director at AstraZeneca...
Read article

October 18, 2017
Webinar Replay: Phase 2 Trial Designs using Program-level Simulations
Cytel's new Trial Innovations Webinar Series provides a platform for the most promising new statistical approaches...
Read article

July 26, 2017
Are Adaptive Designs the Answer to Oncology Development Success?
Sadly, clinical development of anti-cancer therapeutics faces particularly high rates of failure, even in the context...
Read article

July 11, 2017
Collaboration Brings Success for the UK Adaptive Designs Working Group.
The Adaptive Designs and Multiple Testing Procedures Workshop (ADMTP), the first joint meeting of the Adaptive Designs...
Read article
July 6, 2017
When Biostatisticians Disagree About Ethics
By Esha Senchaudhuri The ethical benefits of adaptive clinical trials have been widely acclaimed: higher prospects for...
Read article

May 22, 2017
Jim Bolognese named 2017 American Statistical Association Fellow
James (Jim) Bolognese, Senior Director, Strategic Consulting, Clinical Services at Cytel Inc. was named a 2017 fellow...
Read article

April 25, 2017
Critical Operational Considerations for Interim Analyses
At a recent conference Adam Hamm, Director Biostatistics at Cytel, presented his thoughts on Best Practices and...
Read article

April 11, 2017
FDA 22 Case Studies and Mitigating Phase 3 Risks
In a January 2017 paper (1), the FDA reviewed 22 case studies where promising Phase 2 trials did not result in...
Read article

March 29, 2017
New Publication: Design and Monitoring of Multi-Arm Multi-Stage Clinical Trials
With an increasing interest in platform designs and other innovative designs that involve multiple comparisons over...
Read article

October 7, 2016
Adaptive Designs: A Data Management Perspective
Adaptive designs have the potential to accelerate clinical development, and improve the probability of trial success....
Read article

September 29, 2016
Case studies:Learning from less-well understood adaptive designs
A paper "Best practices case studies for 'less well-understood' Adaptive designs", has been published by the DIA...
Read article

July 18, 2016
Adaptive Design in the limelight with NEJM article
In order for adaptive designs to reach their potential, it’s critical that knowledge is effectively dissemirnated...
Read article

April 28, 2016
Adaptive Designs in Practice
Adaptive Designs in Practice: Interview with NIHR Research Fellow Munya Dimairo NIHR and University of Sheffield...
Read article

April 22, 2016
Dual Agent Dose Escalation Designs
FDA draft guidance on “Co development of two or more unmarketed investigational drugs for use in combination” notes...
Read article

March 11, 2016
EAST takes on Multi-Arm Multi-Stage Designs
There has been increasing interest in multi-arm multi-stage trials with treatment selection and sample size...
Read article
March 3, 2016
Adaptive SSR: Debunking the inefficiency myth
'The aim of a discussion should not be victory but progress.' This principle, expressed by the French essayist Joseph...
Read article
October 1, 2015
It’s Time to Bridge the Gap Between Pharmacometrics and Biostats
This week marks the sixth annual American Conference on Pharmacometrics, held this year in Crystal City, VA. Situated...
Read article
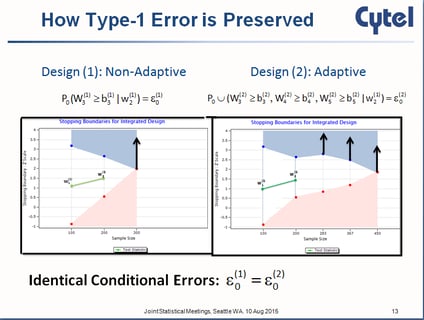
September 17, 2015
Inference on Confidence Intervals for Adaptive Designs: The Latest Breed of Adaptive Clinical Trials
Most people familiar with adaptive clinical trial designs are familiar with those statistical designs that reject the...
Read article

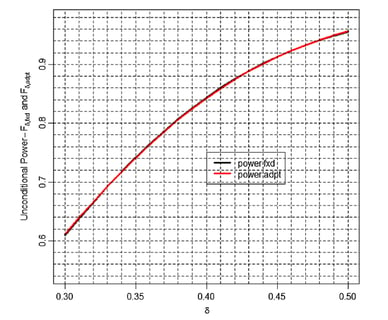
June 26, 2015
Clinical Trials: Why You Should Not Power for Superiority Upfront
Powering a trial for superiority can be financially risky. In some instances it may also prove unnecessary.
Read article
June 11, 2015
Aligning Clinical Development & Regulatory Objectives for Cardiovascular Outcome Trials
When the FDA first began to require pharmaceuticals to perform cardiovascular outcome trials to establish the safety of...
Read article

May 20, 2015
Seamless Adaptive Clinical Trials: What’s really at stake?
Seamless adaptive clinical trials have gained popularity for reducing the projected time it takes to complete the...
Read article

April 30, 2015
New Articles on Adaptive Clinical Trials & Adaptive Financing
Adaptive financing (not to be confused with adaptive licensing) explores how biotechs, pharmaceuticals and potential...
Read article
April 23, 2015
Dose-finding with Sequential Parallel Comparison Designs
Last week the Cytel Blog discussed the benefits of using the Adaptive Maximizing Design [AM Design] for dose-finding...
Read article
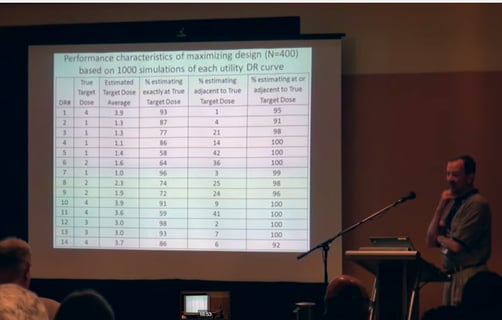
April 16, 2015
Phase 2 Designs for Clinical Utility Limiting Therapies
When testing certain types of new drugs it is known in advance that the adverse side-effects of the medication will...
Read article
March 20, 2015
How Bayesian Strategies Can Expedite a Pediatric Clincial Trial Time by 20 - 40%
Sometimes a new candidate drug for a pediatric study has already been tested on adults for safety and efficacy. We know...
Read article
January 29, 2015
Adaptive Design and Bayesian Statistics: 5 Years Later (Podcast)
February 2015 marks the five year anniversary of the FDA’s Guidance on Adaptive Design Clinical Trials for Drug and...
Read article
December 18, 2014
Early Phase Development Strategy: Bayesian Methods for Go/No-Go Rules
Earlier this week, we at Cytel enjoyed a riveting in-house discussion on the uses of Bayesian decision rules for...
Read article
November 12, 2014
Ranking Adaptive Dose-Finding Designs using Clinical Utility Functions
Clinical utility functions provide Phase 2 trial sponsors with an intuitive metric by which to measure the quality of a...
Read article
September 4, 2014
Adaptive Designs for Evidence Based Oncology: Insights from the Experts
Imagine if we were to count the number of possible reasons that investigators might have for monitoring a biomarker...
Read article

July 29, 2014
Why Using Adaptive Designs Can Attract Investors to Your Trial
Adaptive designs are the unsurprising hot topic of this year’s Joint Statistical Meeting, which features over one...
Read article
July 8, 2014
5 Reasons to Invest in Adaptive Designs for Population Enrichment
The above graphic is from Cyrus Mehta's slides on 'Adaptive Population Enrichment for Oncology Trials with Time to...
Read article
June 5, 2014
Powering Oncology Trials for Success: Adaptive Designs in East
Charles Liu, PhD, Cytel Statistician and Product Manager In the US, cancer is the most common cause of death after...
Read article


