Design and Data Considerations from Cardiovascular Pilot Investigation

Cytel is conducting two pilot projects on head-to-head comparisons using real world data. These projects in oncology and cardiovascular will occur in real time and will take place across our latest webinar series on the topic. The aim of this series is to introduce our audience to head to head to comparison using Real World Data (RWD) while focusing on practical application and results from the pilot projects.
The second webinar from this series was held on July 28, 2020 and outlines the design of the cardiovascular pilot investigation. None of the existing randomized trials of recently developed second-line antihyperglycemic agents can provide adequate information on their comparative effectiveness and safety regarding cardiovascular outcomes. Conducting Target Trials to get information of interest would be costly, difficult to perform, and would take many years to complete. As a result, we need to use observational databases to emulate it. This blog provides a brief on the design of the cardiovascular pilot project. Our team of experts also discussed the data requirements and the data source to be used in the pilot investigation, with the primary challenge focusing on how to assess if data are sufficient for the purposes of trial emulation. Continue reading the blog for a summary of the webinar.
Get access to the webinar recording and download the slides by clicking the button.
This webinar was together presented by Alind Gupta from Cytel, Professor Miguel Hernán from Harvard University, and Judsen Schneider from Nashville Biosciences. You can read more about our speakers here.
Why emulate randomized clinical trials?
Randomized clinical trials are extensively used for comparing the effectiveness and safety of interventions. The Randomized design by itself reduces selection bias as well as considerably reduces other types of biases that are likely to arise in other kinds of designs. As an outcome, randomized trials provide causal estimates of effects which we would not arrive at if we try some type of predictive modeling. Decision-making and health policy need to be informed by causal knowledge about comparative effectiveness and safety of different interventions.
However, randomized trials are not always feasible, and they are often not available for all kinds of questions that we may need to ask. They can be expensive, sometimes unethical, and often impractical and untimely. There are also questions about Real-world generalizability of a randomized trial. For example, due to the eligibility criteria mentioned in the trial, the target population may not appropriately represent the wider population. Hence, the effects that we estimate from such a randomized trial cannot be generalized to the wider population. Moreover, decisions are required to be made even in the absence of a randomized trial to address them.
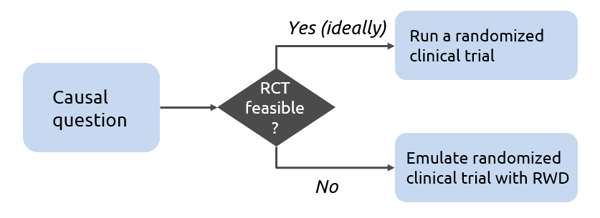
In such circumstances, we can try to emulate a hypothetical randomized clinical trial using real-world observational data. Target trial emulation is the application of design principles from randomized trials to the analysis of observational data, thereby explicitly tying the analysis to the trial it is emulating. We begin with a causal question and check the feasibility of a randomized trial. If it is not feasible, we can emulate a randomized clinical trial with RWD and answer the question by using the target trial framework. Step 1 is to ask a causal question which is equivalent to specifying the protocol of the analogous randomized trial explicitly. Step 2 is answering that causal question and involves identifying a suitable real-world data set and then trying to emulate the randomized trial using RWD. Usually, it is necessary to cycle between steps 1 and 2 iteratively to tune them due to practical limitations, e.g. by restricting eligibility criteria.
Why use a target trial framework?
There are two reasons why we choose to use a target trial framework and not simply analyze the real-world data. The first reason is that we may end up asking the wrong question or a poorly framed causal question. In target trial framework, the explicit representation of the causal question as a target trial prevents this from happening. This webinar specifically includes discussions around defining the question correctly and then answering it accurately. We may end up answering the causal question incorrectly by not accounting for various biases that may arise, not adjusting for relevant confounders or adjusting where we should not, or by using inappropriate methods. As a result, we get biased effect estimates and reach the wrong conclusions. Watch the webinar recording to understand this better through an example presented by Alind and Miguel.
Benefits of using target trial emulation
Target trial emulation can be used to generate efficacy or safety evidence for conditional regulatory approval or post-market assessment. It provides a comparison when network meta-analysis is not possible, for example, if we have disconnected networks. Target trial emulation also helps in expanding the scope of a randomized trial, refining aspects of an existing treatment protocol and enabling a comparison to identify optimal treatment regimen.
Cardiovascular Pilot Investigation
One of the two pilot projects being pioneered by Cytel is a Head-to-head comparison of second-line therapies for type 2 diabetes. During the webinar, the speakers explicitly discussed the considerations for protocol design and data source. Questions such as, how you define a protocol to answer a causal question, how is it different from designing a randomized trial, what is a suitable data source, and others were addressed.
Patients with type 2 diabetes usually start out on metformin as a first-line therapy. But, a lot of patients often fail to achieve or maintain enough glycemic control and require second-line therapy. Currently, there are around ten classes of anti-diabetic drugs that have been approved for use without a head-to-head comparison of effectiveness and safety. The choice of second-line agent varies across clinical practices. While many randomized trials have been or are being conducted to help guide clinical decisions about second-line treatments for diabetes, important questions remain about their comparative effectiveness and safety. Hence, there is a need for comparative effectiveness evidence to guide the choice of second-line therapy to reduce detrimental effects, improve cost-effectiveness and to guide personalized therapy. Once we have the protocol set up, we need to look at data and identify a suitable dataset that will help us successfully emulate a randomized trial. Ideally the data should be a combination of electronic medical records, claim data, clinician notes and other to give us a full picture of the patient history. Nashville biosciences have partnered with Cytel and bring to the table their RWD expertise.
With this pilot project we are looking at initiation of second-line therapy within 12 months of persistent inadequate HbA1c control with metformin use from one of the following treatment arms:
- GLP-1 RA
- SGLT-2 inhibitor
- DPP-4
- Sulfonylureas
- (TZD, insulin)
The other treatment strategies will depend on the data.
Watch the webinar recording and access the presentation slides to get more insights on the project.


