Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

February 17, 2021
The COVID-19 Pandemic prompted the rapid surge in the generation of clinical data that has been scattered across...
Read article
February 1, 2021
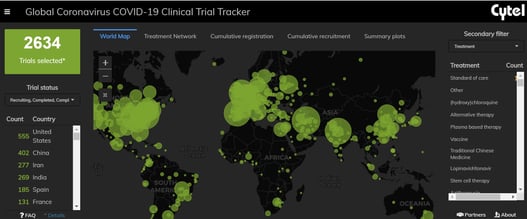
February 2021: Updates from the CYTEL COVID-19 Trial Tracker
Cytel’s COVID-19 Trial Tracker continues to provide real time updates to the status of COVID-19 clinical trials...
Read article
January 11, 2021
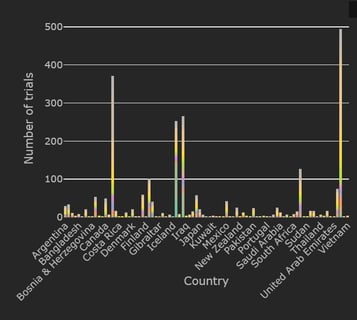
COVID-19 Trial Tracker Updates (January 11)
In April 2020, Cytel launched an open-access global COVID-19 Clinical Trial Tracker to help facilitate greater...
Read article
December 8, 2020
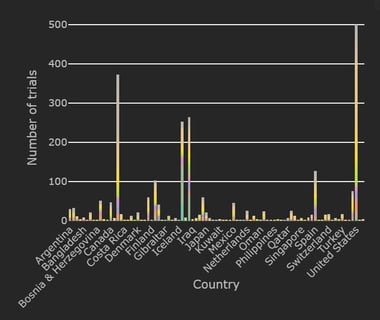
COVID-19 Trial Tracker Updates (December 8)
The Cytel COVID-19 Trial Tracker brings you an up to the minute, real time dashboard about COVID-19 trials around the...
Read article
December 1, 2020
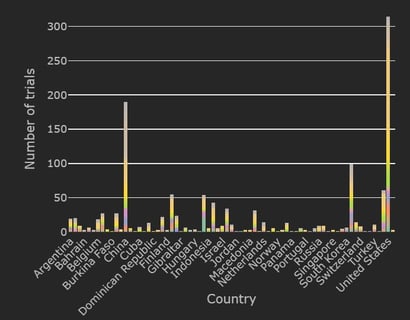
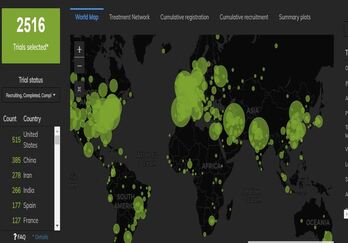
Mapping the Landscape of COVID-19 Clinical Trials in the US
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread. As...
Read article

November 24, 2020
Cytel and Ingress Health at Virtual ISPOR Europe 2020
Virtual ISPOR 2020, held November 16 to 19, presented new opportunities for scientific interaction amongst HEOR...
Read article

September 28, 2020
Advantages of platform designs for investigating COVID-19 therapies
Cytel has recently designed and implemented the TOGETHER Trials, funded by the Bill & Melinda Gates Foundation to...
Read article

September 21, 2020
Novel Adaptive Platform Trial for COVID-19 Therapies
Cytel has designed and implemented a novel adaptive platform trial for early stage COVID-19. The severity of the...
Read article

June 29, 2020
The Good Data Submission Doctor: CDISC for COVID-19
From the time the COVID-19 outbreak was declared a pandemic, the number of studies conducted around the world to either...
Read article
May 22, 2020
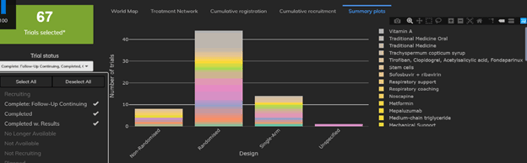
4 Things You Need to Know about COVID-19 Trial Designs
The Cytel Trial Tracker now features summary plots that display trials by country, trial status and study design. This...
Read article
May 14, 2020
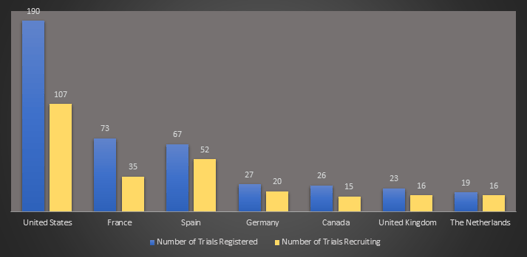
Weekly COVID-19 Trial Tracker Updates
This has been an exciting week for COVID-19 studies. We learned that several Cytel clients who have designed new...
Read article

May 7, 2020
COVID-19: Trials, Designs and Tools for Promising Results - A Virtual Panel Discussion
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread...
Read article


