Mapping the Landscape of COVID-19 Clinical Trials in the US
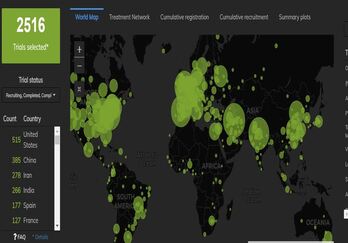
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread. As several entities develop curative and preventive responses against COVID-19, alignment with regulatory recommendations is key for developing effective and safe intervention. Moreover, fast regulatory approval will translate into early availability of interventions to address unmet needs.
Continue reading to get an overview of the registered COVID-19 clinical trials landscape, with a story on the special attention received by Hydroxychloroquine treatment.
Background
Cytel and Ingress Health (now a Cytel company) conducted a study aimed to describe the landscape of the registered COVID-19 clinical trials in terms of trial designs, interventions and endpoints. The core of the analysis was a mapping of the ongoing curative and preventive intervention trials on COVID-19 against the guidelines provided by FDA. Data on trial characteristics were extracted from Cytel Global Coronavirus COVID-19 Clinical Trial Tracker and complemented by other databases (e.g. clinicaltrial.gov). Since the study was largely restricted to these two sources, it does imply a limitation with regards to fully covering the information available in trial protocols.
The scope of the review included Phase II and III trials in the US, and the timeframe considered was until June 8, 2020 for Drug trials and September 30, 2020 for Vaccine trials.
Analyses of Curative and Preventive Drug Trials
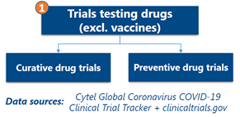
In Curative Drug trials, the study showed fragmentation in tested interventions as we saw a high proportion of trials in the “Other” category (including kinase inhibitors, immunomodulators, anticoagulants, antiparasitics, combinations therapies). Monoclononal antibodies are the most tested curative interventions. For preventive drugs, Antimalarials was the most tested intervention. Overall, the study of drug trials showed gaps in assessing healthcare resource utilization (for example, need for ICU, hospitalization, mechanical ventilation), which are relevant for developing system level response to the virus.
The Rise and Fall of Hydroxychloroquine (HCQ) Intervention
One of the major curative drugs tested in several trials was of course hydroxychloroquine. The efficacy and safety of hydroxychloroquine (HCQ) for the prevention and treatment of COVID-19 received great attention over the last several months. Laboratory studies and case series published early in the pandemic supported its efficacy. The scientific community raced to conduct observational and randomized evaluations of the drug in all stages of the disease, including prophylaxis, early treatment, and advanced disease. Yet a divisive media response affected recruitment, funding, and subsequent enthusiasm for continuing scientific investigations.
Of the more than 300 HCQ trials registered, fewer than 50% report having recruited any patients, and most trials might fail to achieve any useful portions of their intended sample size. Multiple observational studies and two large randomized trials have demonstrated HCQ does not offer efficacy against COVID-19 in hospitalized patients. Prophylaxis studies and early treatment studies provided heterogeneous results and are plagued by low event rates and poor study outcome monitoring. Emerging high-quality evaluations of prophylaxis and early treatment do not support a role for HCQ in these populations.
Most notably, the enthusiasm for HCQ has been one of politicization rather than science. The story of HCQ for COVID-19 followed a pattern of initial enthusiasm supported by poor quality evidence, followed by disappointment based on more rigorous evaluations. This experience in the COVID-19 era calls for the depoliticization of science away from media glare.
Analyses of Vaccine Trials
For the four major vaccine trials, we observed that there were clear gaps in targeting children and (potentially) pregnant women. However, these four trials were aligned with the FDA recommendations regarding most relevant efficacy endpoints. We observed some unclarity on alignment with FDA severity definition in two cases.

Cytel is helping our clients by providing recommendations for completing data collection, handling missing information, modifying protocols and amending statistical analysis plans. In addition, Cytel’s software is helping customers more efficiently design adaptive trials for COVID-19, more accurately analyze small or skewed datasets, and forecast future enrollment for all ongoing trials in the face of so much uncertainty.
Click on the button to learn about our solutions.


