In April 2020, Cytel launched an open-access global COVID-19 Clinical Trial Tracker to help facilitate greater collaboration between researchers, policymakers, clinicians, journalists, philanthropists, and other critical stakeholders. Funded in part by The Bill and Melinda Gates Foundation, a leader in global health solutions, this live dashboard offers an overview of all the clinical trials taking place in the international effort to tackle the pandemic.
We have been posting regular updates on the clinical development of COVID-19 therapy and vaccines, on Cytel’s Blog page. The following details are based on an updated data search accessed on January 11.
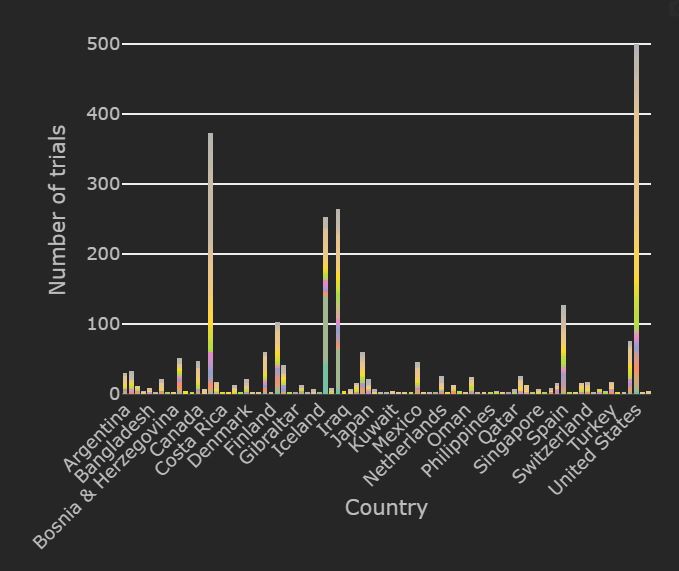
There are currently 2533 registered interventional trials worldwide which include 343 for hydroxychloroquine, 111 for lopinavir/ritonavir, 94 using stem cell therapies, 93 for Azithromycin therapies, 165 for plasma based therapies and 70 for tocilizumab.
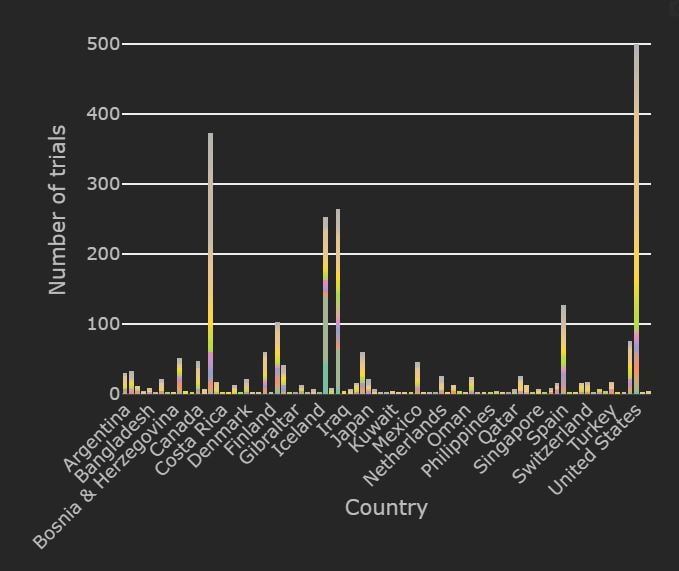
The Clinical Trial Tracker shows that 323 trials have completed thus far:
- 232 in Iran
- 24 in China
- 17 in United States
The number of trials conducting patient recruitment globally has dropped to 1274. Most of these trials are based in the United States, China and Spain. The top therapies recruiting in the United States are Plasma based therapy, mAb and hydroxychloroquine. In Europe, majority of the patient enrollment is for hydroxychloroquine, Tocilizumab and vaccine trials.
Click below for more information on the Cytel COVID-19 Trial Tracker.

Whether you are planning on designing a clinical research program for COVID-19 or looking to overcome challenges that the virus has created for your current trials, the next steps are vital. Cytel’s experienced team of statisticians and data scientists can work with you to identify any potential challenges and complications you may be facing.
Cytel provides:
- Meta-analysis and modelling using Real-World Evidence
- Consultations on innovative clinical trials
- Bayesian network analysis
- Synthetic and External Control Arm Construction
- Real World Data through the COVID-19 Trial Tracker