Cytel's blog featuring the latest industry insights.

The Cytel Trial Tracker now features summary plots that display trials by country, trial status and study design. This enables us to take a deeper dive into patterns of trial design in COVID-19 studies. This blog post explores the use of single-arm studies, numbers of multiarm studies, and more.
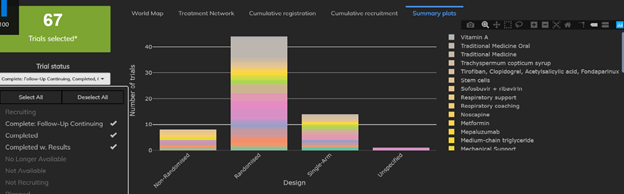
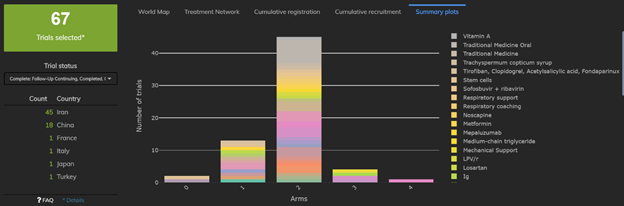
1: The Proportion of Single-Arm Trials Appears to Be Decreasing
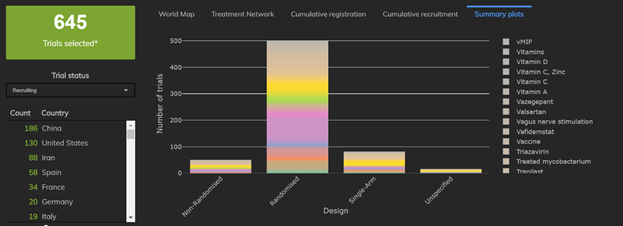
There are currently 67 trials already completed and 645 trials currently recruiting. While the vast majority of completed trials were randomized two-arm trials, we quickly see that nearly 21% of trials were in fact single-arm studies. By contrast, fewer than 100 of the 645 (trials currently recruiting (15.5%) are single-arm trials. The proportion of single-arm trials has fallen by about 5% as information about the disease accumulates.
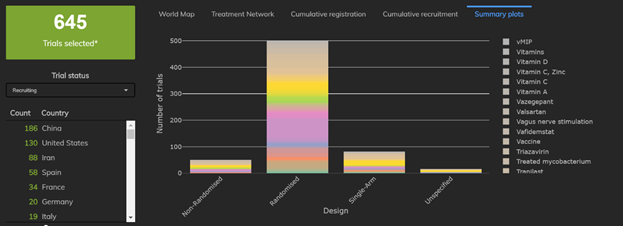
2: The Number and Proportion of Multiarm Trials is Rising
Fewer than 10 of the 67 trials completed had 3 or more trial arms, whereas more than 100 of the 645 trials currently recruiting have 3 or more trial arms. Particular trials to note are the 13-arm Hydroxychloroquine Trial and a 9-arm trial testing both Hydoxychloroquine and Lopinavir.
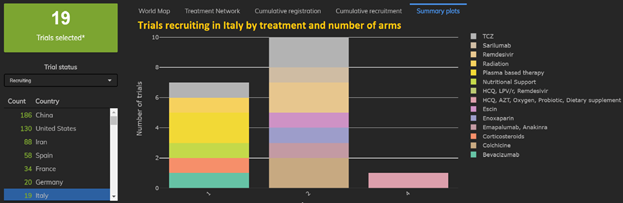
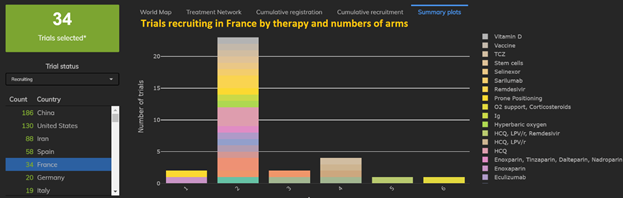
3: Amongst EU Nations, France takes the lead in proportions of multiarm trials and Italy takes the lead in proportion of single-arm studies.
7 out of 19 trials in Italy are single-arm trials, whereas 8 out of 34 trials in France have three or more arms.
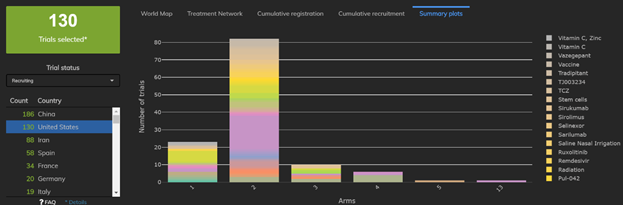
4: Only 63% of Trials in the United States have a traditional 2-arm design
The United States is also moving away from the traditional 2-arm design. Only 80 of 130 trials currently recruiting have two arms, while 23 of these trials are single-arm studies. The remainder have three ore more arms.