Cytel's blog featuring the latest industry insights.

This has been an exciting week for COVID-19 studies. We learned that several Cytel clients who have designed new clinical trials using our East software, are about to begin enrolling. Our subject-matter experts are also heavily involved in designing vaccine trials, as well as offering biometrical support and data management for three vaccine trials that have already begun to enroll.
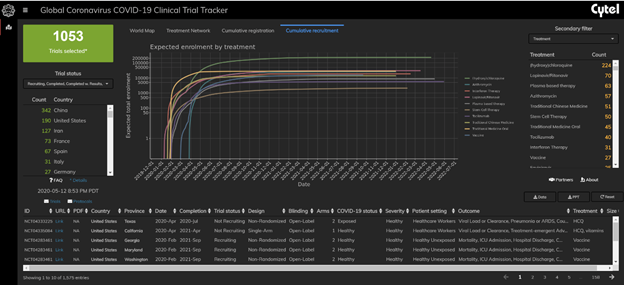
There are now 1053 registered trials
Over 50% of registered trials are small trials with fewer than 100 patients. These are overwhelmingly traditional trials with two arm designs. There also appear to be a lot of duplicate trials across countries. For example there are still nearly 200 chloroquine and hydroxychloroquine trials recruiting.
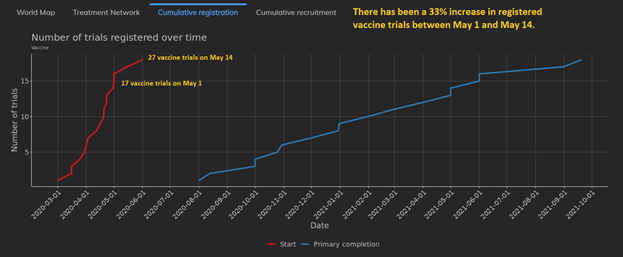
Vaccine trials have increased by nearly 33% since the beginning of May. There are now 27 vaccine trials with 15 recruiting, whereas on May 1 there were 17 vaccine trials with 10 recruiting.
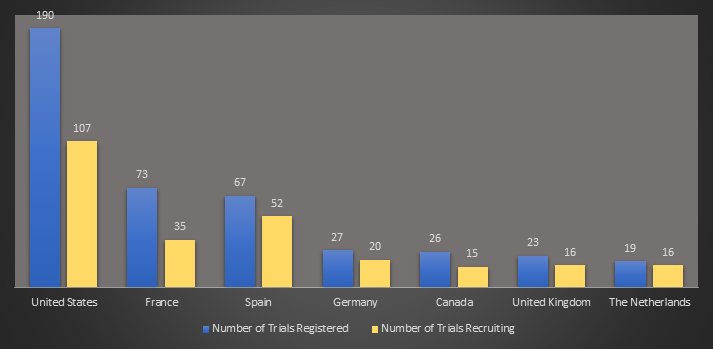
The numbers of therapy trials are also increasing in every region. There are now 219 registered trials in the European Union with France taking the lead with 73 trials, and Spain a close second with 67 trials. Spain leads the EU in the number of trials actively recruiting with 52 trials to France’s 32.
Additionally the United States has 190 registered trials with 107 recruiting, and the United Kingdom has 23 trials with 16 recruiting.

For more information on the Cytel COVID-19 Trial Tracker click below.
References and Further Reading:
