The combination of greater access to electronic health records, bigger electronic claims datasets, and the need for more clinical insight in ensuring patient safety, has made observational studies an important new tool in trial design. Observational studies typically take non-randomized data from outside of a trial and use quantitative and modeling techniques to draw conclusions from big datasets. While typically used for HEOR and market access, augmenting regulatory submissions with observational studies is gaining prominence. As with all data analyses, there is an implicit rule of ‘garbage in-garbage out,’ where data that is not up to the standard required for the formation of sound scientific judgment, should not be used. Sponsors should rely on the most sophisticated tools and advanced analytics to make the most rigorous use of available data.
Uses of Observational Studies for Regulatory Approval
Observational studies can be used throughout the product development journey, although popularly used for market access. Prior to market access, FDA and EMA are increasingly allowing observational studies to augment the findings of randomized control trials as they prepare for regulatory submission. A traditional trial might have shown safety or effectiveness, but more data might provide a stronger claim than what was found during a clinical trial. Statisticians can help sponsors determine when this is the case and identify opportunities for strengthening regulatory portfolios through observational studies.
This feature of observational studies also highlights the necessity of working with statisticians, epidemiologists and others who can be trusted to use data with integrity, rather than to create arbitrary analyses that lead people towards believing what the data clearly does not say. For example, if there is a bias in the population or problematic data collection, a sophisticated but accountable statistician will help sponsors extract evidence without methodology that unfairly biases the findings in favor of the sponsor. Empirical objectivity must always remain a key aspect of observational studies.
Proving Commercial Value through Indirect Treatment Comparisons
Observational studies also play a key role in market access. Several kinds of observational studies are used to prove commercial value after a new therapy or device has already been approved. One popular use that requires significant statistical support are indirect treatment comparisons. Suppose a sponsor has a new medicine that has been tested against a standard of care, but in fact upon trying to enter the market, he or she finds that there are several other medicines available. Call these new therapies, Therapy A, Therapy B, Therapy C, and so forth. Rather than performing individual pairwise comparisons of them all, the sponsor needs only to show that the new therapy is more effective than say Therapy A, which has already proven to be more effective relative to Therapy B and Therapy C. Assuming that these three therapies have already been compared against each other, the sponsor can glean outcomes about relative effectiveness against Therapy B and Therapy C after only conducting a comparison to Therapy A.
Exploring New Research Avenues
Another use of observational studies is to identify areas where a sponsor’s therapy might work even better than in a trial. For example, suppose a particular subpopulation has specific comorbidities that were not tested during a trial, or that in the market a specific new therapy would work even better in combination with an existing one. These cases are examples of when the new post-regulatory research question, might be slightly different than the one that was studied through the RCT. Therefore, it is imperative to use an experienced statistician for such a study. As the research question changes, even somewhat, the statistical measurements must adjust to the new question. There is typically no straightforward way to do this. Statisticians must adapt on a case by case basis.
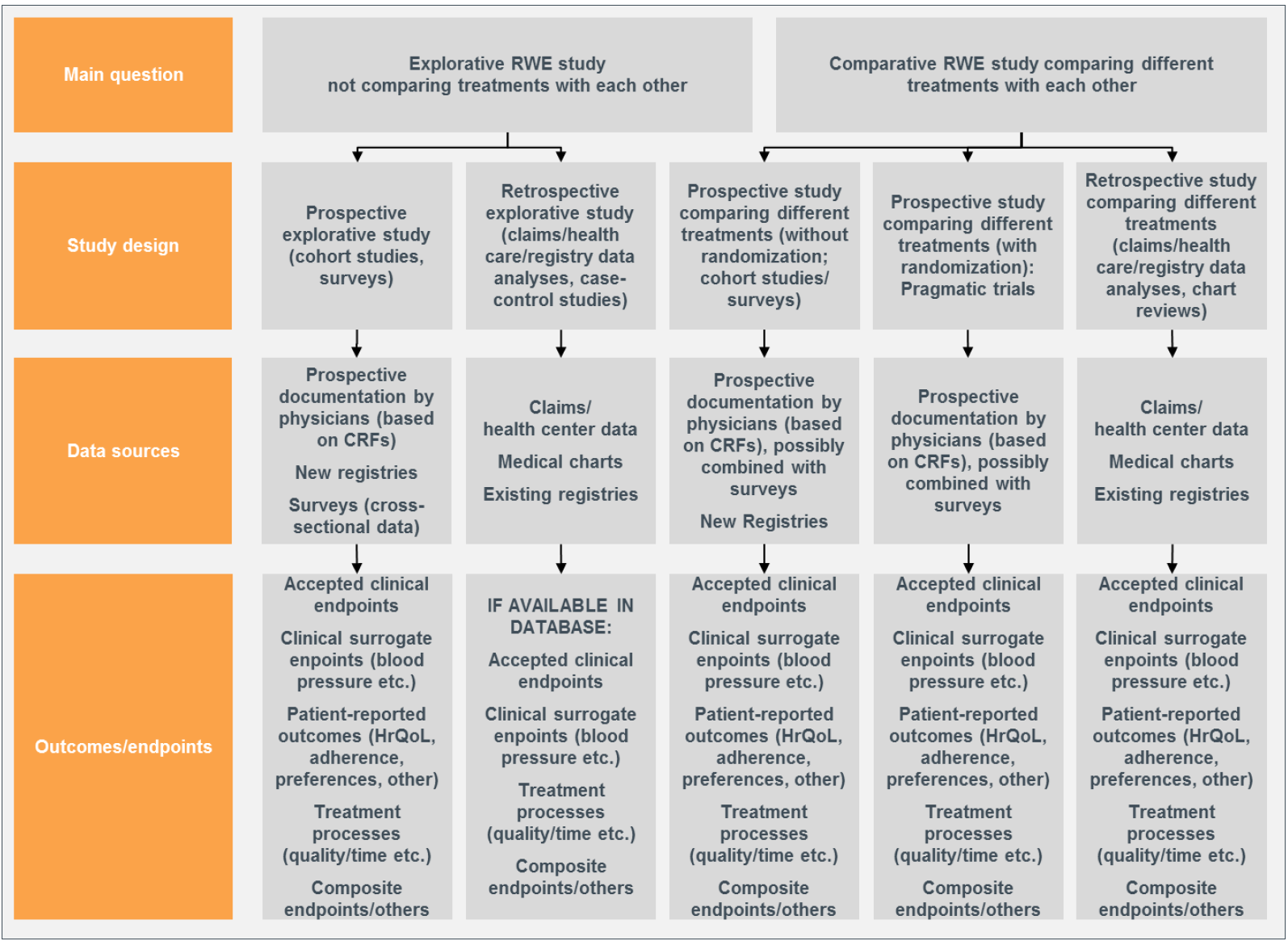
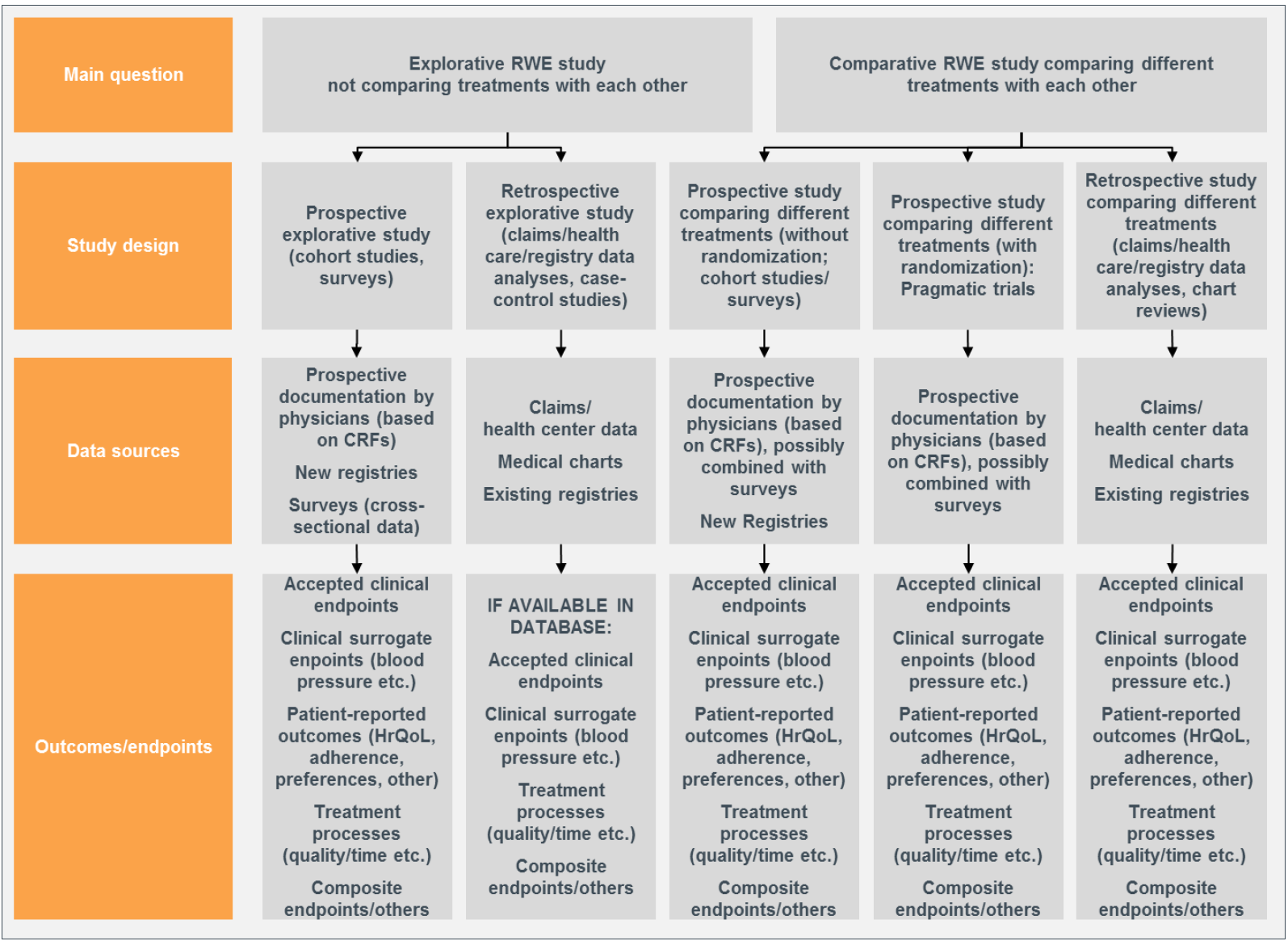
Image Source: 'Real-World Study Planning: A Systematic Approach' by Thomas Wilke
At Cytel, we generate evidence that complements traditional randomized clinical trials, accelerates submissions, expands indications and supports health-economic decision-making. Cytel focuses on using sophisticated statistical, decision modeling and data science techniques to generate evidence that regulators and payers will trust.
Whether you are a smaller organization seeking to optimize your programs, or a larger organization developing an RWE strategy to support a regulatory submission, we can help. Our team can leverage real-world data to support smarter clinical development.
Contact us to learn more and schedule a meeting with our experts.