
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

October 21, 2021
A number of methods currently exist to measure non-adherence and non-persistence of medical therapies, for improved...
Read article

October 7, 2021
Non-adherence and Non-persistence
‘Drugs do not work in patients who do not take them,’ said former surgeon general C. Everett Koop. Unfortunately, the...
Read article

April 28, 2021
Trial Selection: From Art to Science
Recently, Cytel co-founder Professor Cyrus Mehta noted that, “Clinical trial design selection is too much like an art,...
Read article

April 20, 2021
New Dimensions of Clinical Trial Optimization
For much of the past three decades, even as methodologies for clinical trial design have advanced and refined, the idea...
Read article

April 13, 2021
7 Steps to an Evidence Dossier for Wearables
There has been an increasing use of digital measures in drug development recently. New wearables technologies can help...
Read article

April 7, 2021
Career Perspectives: Interview with Neha Sati
Scientists at Cytel recently published a paper in the Journal of the American Medical Association (JAMA). Among the...
Read article

March 31, 2021
Wearables and Decentralization
As decentralized clinical trials become more attractive in an era of COVID-19, the role of wearables in clinical...
Read article

March 26, 2021
Using External Evidence for Decision-Making for Medical Devices
Former Commissioner of the FDA, Dr. Scott Gottlieb, in several public presentations, would bemoan missed chances to...
Read article

March 19, 2021
5 Steps to Regulatory Success with Wearables Designs
The use of wearable and digital technology requires considerations for both drugs and devices regulations, and...
Read article

March 18, 2021
Computing & Statistics: Are You Ready for the Industry Transformation?
Over the past ten years High-Performance Computing (HPC) has transformed medical research through advances in genomics,...
Read article

March 12, 2021
Wearables: Translating raw data to actionable information
With wearables likely to become a regular part of clinical trial design, statisticians could benefit by familiarizing...
Read article

March 11, 2021
Data and analysis in Modern Oncology Clinical Development
In the recent years, Oncology trials are seeing a technological shift that is expected to make them faster and more...
Read article

March 9, 2021
Seeing Uncertainty: New Frontiers of Statistical Communication
When statistical sciences were in their infancy, the communicative benefits of statistics were widely touted. Thousands...
Read article

February 25, 2021
Use of Wearables in Confirmatory Clinical Trials
The convergence of several distinct trends has made wearables an increasingly attractive option for use in confirmatory...
Read article

February 19, 2021
Selecting Your Next Clinical Trial Design Using Quantitative Methods
C-Suite and R&D Decision-Makers are always striving to make evidence-driven decisions. Yet the rules by which evidence...
Read article
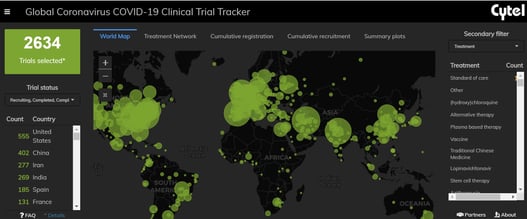
February 17, 2021
An Interview with Louis Dron on the Benefits and Future of Cytel’s Trial Tracker
The COVID-19 Pandemic prompted the rapid surge in the generation of clinical data that has been scattered across...
Read article

February 11, 2021
The biostats and clinical overview of a growing clinical strategy
The past two years have witnessed a heightened interest in the use of wearables in clinical development. The unexpected...
Read article

February 9, 2021
New Meta-Analysis in JAMA Uses Novel Quantitative Techniques to Demonstrate Baseline Characteristics Informing Response to Common Therapy for Kidney Cancer
Recent years have witnessed improving survival outcomes for those struggling with a range of common kidney cancers....
Read article

February 4, 2021
Simulation Based Clinical Trial Optimization
The past decade has witnessed the rise of simulations-based clinical trial optimization in a manner unimaginable to...
Read article
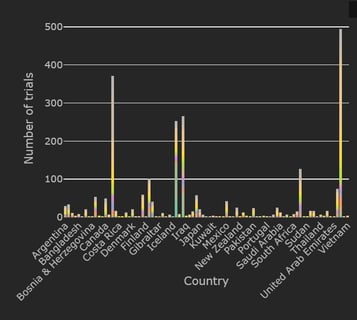
February 1, 2021
February 2021: Updates from the CYTEL COVID-19 Trial Tracker
Cytel’s COVID-19 Trial Tracker continues to provide real time updates to the status of COVID-19 clinical trials...
Read article
January 29, 2021
Computation and Clinical Trial Design: New Directions
Historically, advances in the statistical design of clinical trials have accompanied progress within the science and...
Read article

January 22, 2021
The Role of Real World Evidence after COVID19
COVID-19 has transformed the pharmaceutical industry in a manner that few could have predicted only a year ago. One of...
Read article
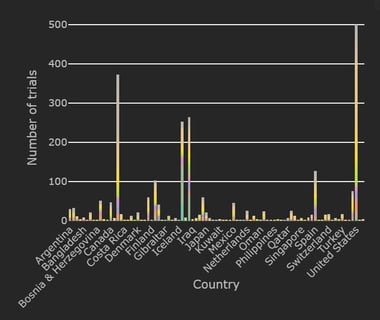
January 11, 2021
COVID-19 Trial Tracker Updates (January 11)
In April 2020, Cytel launched an open-access global COVID-19 Clinical Trial Tracker to help facilitate greater...
Read article

December 21, 2020
Year-End Roundup: Your Favorite Blog Posts of 2020
2020 has been an unusually difficult year as the global pandemic impacted all of our lives. This year, the Cytel blog...
Read article

December 17, 2020
2020 Recap by Yannis Jemiai, Chief Scientific Officer, Cytel
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article
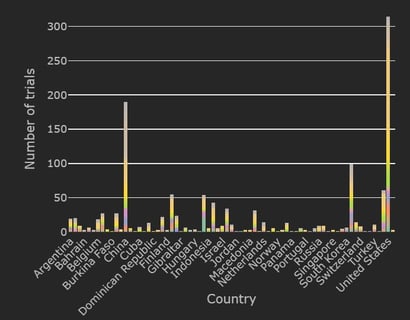
December 8, 2020
COVID-19 Trial Tracker Updates (December 8)
The Cytel COVID-19 Trial Tracker brings you an up to the minute, real time dashboard about COVID-19 trials around the...
Read article
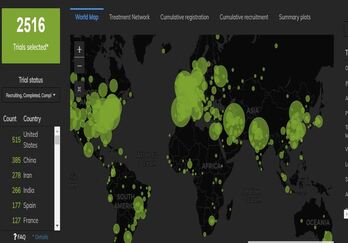
December 1, 2020
Mapping the Landscape of COVID-19 Clinical Trials in the US
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread. As...
Read article

November 24, 2020
Cytel and Ingress Health at Virtual ISPOR Europe 2020
Virtual ISPOR 2020, held November 16 to 19, presented new opportunities for scientific interaction amongst HEOR...
Read article

November 19, 2020
10 Key Qualifications for Independent Statisticians Reporting to the DMC
Data Monitoring Committees (DMCs) are groups of independent experts who periodically receive (by-arm) reports created...
Read article

November 12, 2020
Join Cytel and Ingress Health at Virtual ISPOR Europe 2020
Cytel and Ingress Health (now a Cytel company) will be contributing to a range of events at Virtual ISPOR EUROPE 2020,...
Read article

November 5, 2020
Role of RWA in Transforming Oncological Research
In oncology, many manufacturers go into niche indications, where there are very specific tumors, and then they opt for...
Read article

November 3, 2020
Staffing Needs for RWE Delivery
When an expert statistician is paired with an experienced set of data managers, opportunities to capitalize on...
Read article

October 22, 2020
An Interview with Bart Heeg (Part 2): New Trends in HEOR
In this two-part blog series, we interview Bart Heeg, Vice President HEOR and Founder at Ingress Health (A Cytel...
Read article

October 20, 2020
Interview with Thomas Wilke: Health Economics/World Evidence Studies
In this interview with Thomas Wilke, Principal Scientist at Ingress-Health (a Cytel company), we talk to him about his...
Read article

October 19, 2020
The Uniqueness of COVID-19 Data Challenges; The COVID-19 trial tracker
COVID-19 has created extreme uncertainties -- a dearth of historical information combined with the need for safety,...
Read article

October 14, 2020
The Increasing Importance of Health Economics
A credible evidence base is needed to support and document the economic value of new technologies and therapeutic...
Read article
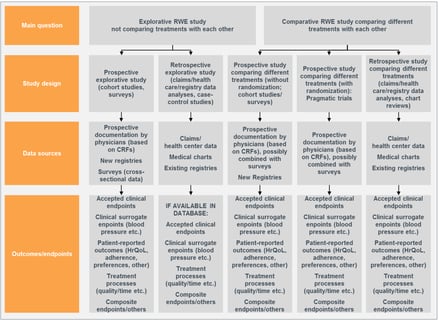
October 13, 2020
Introducing Observational Studies – Three Trends for Statisticians
The combination of greater access to electronic health records, bigger electronic claims datasets, and the need for...
Read article

October 7, 2020
RWE Needs for Natural History Studies
With the rise in digital technologies, there has been an explosion in the volume and type of data sources. We can...
Read article

September 25, 2020
Use of External Controls in Clinical Development – Download Audiobook
Regulators in both the United States and Europe have responded positively to the use of synthetic control arms (SCA)s...
Read article

September 16, 2020
New Audiobook on Synthetic Control Arms
Synthetic control arms (SCA) are virtual trial arms that use historical claims data and observational data to simulate...
Read article

June 22, 2020
Webinar: Synthetic and External Controls in Clinical Trials
Cytel scientists recently published a new eBook on synthetic control arms and a new scientific primer for the more...
Read article

June 8, 2020
New Primer and Ebook on Synthetic Control Arms
Cytel has recently published a new ebook on synthetic control arms, and a new scientific primer as well.
Read article

May 12, 2020
Oncology Trial Design & Development Webinar Series
In our previous blog, “Remote Working Arrangement – How to get it right?”, we talked about how the need for social...
Read article

May 7, 2020
COVID-19: Trials, Designs and Tools for Promising Results - A Virtual Panel Discussion
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread...
Read article

March 5, 2020
Managing risk in clinical development: Is your data strategy fail-safe?
Generating high-quality clinical data is a vital but challenging task in modern drug development. Unfortunately, in the...
Read article

February 20, 2020
Unlock the power of your clinical data with these five top tips
It is widely acknowledged among drug developers that one of their most important assets is the data generated during...
Read article

February 6, 2020
Is your data strategy set up to tackle key challenges in early clinical development?
In clinical development, a high-quality evidence package is a prerequisite for a new therapy to gain approval from...
Read article
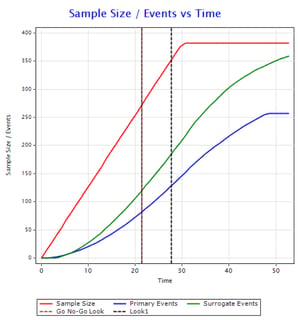
January 30, 2020
Designing Event-based Studies: Interview with Pantelis Vlachos
The Cytel Trial Design Innovations (CTDI) Webinar Series recently hosted a webinar on designing event-based studies....
Read article

January 22, 2020
What could you accomplish with a fresh approach to your clinical data strategy?
In the quest for clinical success, we all strive for evidence packages of the highest quality. If the clinical data is...
Read article

January 16, 2020
Adaptive Population Enrichment in a Phase III Oncology Trial
January’s Cytel Trial Design Innovations (CTDI) Webinar Series will feature Biostatistician and pioneering Bayesian...
Read article

January 8, 2020
How to optimize your data strategy to drive success in clinical development
In clinical development, data is the vital ‘foundation’ that supports your programs. To successfully bring a promising...
Read article

December 18, 2019
Year-End Roundup: Your Favorite Blog Posts of 2019
With only two weeks left for this fabulous year to end, we would like to thank all our blog subscribers and new readers...
Read article

December 10, 2019
Impact of AI on Clinical Development
In association with Statisticians in the Pharmaceutical Industry (PSI) , UCB and Cytel hosted a symposium on September...
Read article

November 27, 2019
Interview with David Kerr: Data Monitoring Committees (DMCs) – Behind Closed Doors
At the 2019 Challenges in Rare Diseases Clinical Trials Symposium and East training, Cytel partnered with Alexion to...
Read article
October 10, 2019
The Challenges of Rare Diseases in Clinical Trials Symposium and Hands-on East Training
A disease is generally considered to be rare if it affects one patient per 200,000 people (1) and most rare diseases...
Read article

March 20, 2019
Operation Rescue: Addressing Lagging Trials
No one plans to have a trial whose data collection needs rescuing. However, lagging enrollment rates, operational...
Read article
January 10, 2019
Podcast: Overcoming Phase 1 Development Challenges
Nand Kishore Rawat is a Director and Head, Early Phase Biostatistics based in the King of Prussia, PA Cytel office. We...
Read article

December 5, 2018
Creating a Common Language: Forging Statistical and Clinical Collaborations
In this blog, Paul Terrill, Director of Strategic Consulting at Cytel outlines his blueprint for ensuring smooth...
Read article

November 27, 2018
Top 5 Frequently Asked ADaM Questions
This is the third in our blog series ' The Good Data Submission Doctor' in which Angelo Tinazzi, Director of Standards,...
Read article

November 5, 2018
New publication addresses critical issues in ultra-orphan indications
Cytel biostatisticians Cyrus Mehta and Lingyun Liu, together with Charles Theuer, CEO of TRACON Pharmaceuticals have...
Read article

November 1, 2018
Top 5 Frequently Asked SDTM Questions
In this second post of the “Good Data Submission Doctor” ( read my first post The Master Recipe: Quality and Attention...
Read article


October 23, 2018
Career Perspectives: Interview with Munshi Imran Hossain, Senior Data Scientist
Cytel data scientists apply advanced statistical techniques including predictive modeling of biological processes and...
Read article

September 20, 2018
Career Perspectives: Interview with Adam Hamm, Director of Biostatistics
At Cytel we believe that expert statistical input has the power to shape the future of clinical development: de-risking...
Read article

September 5, 2018
Podcast: Enhancing Patient Enrollment Forecasting with EnForeSys 2.0
EnForeSys is Cytel’s tool for patient recruitment planning. We have discussed on the blog recently with Tufts...
Read article

August 23, 2018
Career Perspectives: Interview with Meredith Alm, Manager, QA Compliance
Cytel has grown significantly over the last 30 years, with operations across North America, Europe, and India. All of...
Read article
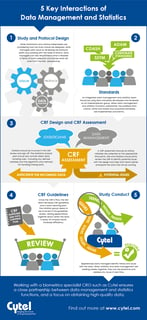
July 27, 2018
Infographic: 5 Key Interactions of Data Management and Statistics
In this blog, we share a new infographic based on this popular blog post illustrating some of the critical interactions...
Read article

July 24, 2018
Career Perspectives: Interview with Sam Hsiao, Associate Director, Strategic Consulting
At Cytel our strategic consulting team works on a wide range of projects including: Identifying the best clinical trial...
Read article

June 28, 2018
Non-Compartmental Analysis and the Early Phase Regulatory Environment
By Esha Senchaudhuri With thanks also to Jitendarreddy Seelam and Ramanatha Saralaya for their input. The fact of the...
Read article

June 19, 2018
The Importance of Standardization in Clinical Outsourcing
At the recent PCMG conference in Malta, Adrian Otte ( Independent Consultant, formerly VP Global Development Operations...
Read article

June 14, 2018
What makes a good data manager?
In this blog, Paul Fardy, Executive Director of Data Management at Cytel shares his thoughts on how the data manager...
Read article

April 25, 2018
Overcoming Data Management Challenges in Immuno-Oncology Trials
Data management is an essential building block for successful Immuno-Oncology (I-O) trials. At the Immuno-Oncology...
Read article

April 13, 2018
Exploring Challenges of Clinical Trial Operations Part 2 with Ken Getz
We return to our discussion with Ken Getz of the Tufts CSDD for part 2 of our blog post on key challenges in clinical...
Read article

April 5, 2018
Ken Getz: Exploring Challenges of Clinical Trial Operations
Photo by J. Kelly Brito on Unsplash Research on clinical trial enrollment makes for sobering reading, characterized by...
Read article

March 16, 2018
Career Perspectives: Interview with Benjamin Esterni, Principal Biostatistician
At Cytel we believe that expert statistical input has the power to shape the future of clinical development: de-risking...
Read article

February 21, 2018
Developing efficient tools for ADaM dataset creation
By Diganta Bose, Statistical Programming Team Lead at Cytel Editor's note: This blog is based on work presented at...
Read article

February 13, 2018
Career Perspectives: Interview with Ursula Garczarek, Associate Director - Strategic Consulting
Our strategic consulting team work on projects such as: Identifying the best clinical trial design, implementing...
Read article

February 6, 2018
Life in Programming: Interview With Ajay Sathe
We were excited to learn recently that Ajay Sathe, the CEO of our India Operations, was awarded lifetime honorary...
Read article


