
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

March 30, 2021
As of today, our Industry has not defined any approach, nor does an official regulatory agency...
Read article

February 24, 2021
Avoiding Lost-in-Translation with Submission Terminology
In a previous post, I discussed the importance of proper use of CDISC Controlled Terminology (CDISC CT) in SDTM....
Read article

January 28, 2021
A little walk in the CDISC Library, hand in hand with SAS
The Christmas break presented an opportunity to make my first concrete steps into the CDISC Library. Overall, it was a...
Read article

December 18, 2020
Submitting Software Programs to the Regulatory Agencies
Can I submit software programs other than SAS? What software programs should I submit? Are sponsors required to submit...
Read article

November 23, 2020
Data Standards and Submission Highlights from PHUSE EU CONNECT 2020
The Virtual PHUSE-EU CONNECT Conference was held from November 8 to 13 and the event was a great success, despite all...
Read article

November 3, 2020
Staffing Needs for RWE Delivery
When an expert statistician is paired with an experienced set of data managers, opportunities to capitalize on...
Read article

October 28, 2020
When your ADaM package is not traceable back to SDTM
About three years ago, Cytel was helping a sponsor on a project where I had to conduct surveillance of some CRO...
Read article

October 20, 2020
Interview with Thomas Wilke: Health Economics/World Evidence Studies
In this interview with Thomas Wilke, Principal Scientist at Ingress-Health (a Cytel company), we talk to him about his...
Read article

October 14, 2020
The Increasing Importance of Health Economics
A credible evidence base is needed to support and document the economic value of new technologies and therapeutic...
Read article
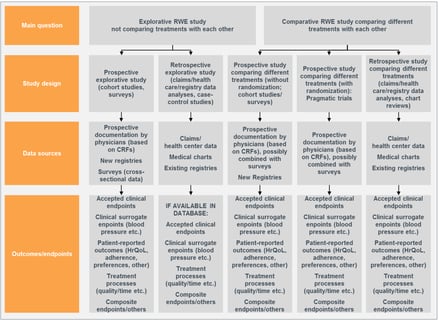
October 13, 2020
Introducing Observational Studies – Three Trends for Statisticians
The combination of greater access to electronic health records, bigger electronic claims datasets, and the need for...
Read article

October 7, 2020
RWE Needs for Natural History Studies
With the rise in digital technologies, there has been an explosion in the volume and type of data sources. We can...
Read article

September 25, 2020
Use of External Controls in Clinical Development – Download Audiobook
Regulators in both the United States and Europe have responded positively to the use of synthetic control arms (SCA)s...
Read article

September 16, 2020
New Audiobook on Synthetic Control Arms
Synthetic control arms (SCA) are virtual trial arms that use historical claims data and observational data to simulate...
Read article

September 10, 2020
Does your Trial need a Synthetic Control Arm?
Single arm trials are emerging as an accepted way of assessing a new treatment intervention. They establish clinical...
Read article

August 7, 2020
Creating a Synthetic Control from Your Natural History Study
Recently a biotech approached Cytel for support with a Phase 2 Study in oncology. Regulators had requested a natural...
Read article

August 5, 2020
Design and Data Considerations from Cardiovascular Pilot Investigation
Cytel is conducting two pilot projects on head-to-head comparisons using real world data. These projects in oncology...
Read article

April 27, 2020
Highlights from the 2020 Virtual CDISC EU Interchange - Part 2
In the first part of this two-parts blog, I speak about how the European CDISC Committee (E3C) together with CDISC...
Read article

April 24, 2020
Highlights from the 2020 Virtual CDISC EU Interchange by Angelo Tinazzi
In early March, when countries around the world started implementing lockdowns, the European CDISC Committee (E3C)...
Read article


