Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

June 14, 2022
Suppose you had to choose six clinical trials intended for registration with regulatory agencies, only six, to explain...
Read article

February 22, 2022
How and Why to Implement Optimal Adaptive Promising Zone Designs
When determining the best possible statistical design for a particular trial, large pharmaceuticals and small biotechs...
Read article

January 27, 2022
Winners of The Promising Zone Quiz
As a part of Cytel’s 10 Year Anniversary of the Promising Zone Design, Cytel hosted a quiz on “Keeping the Promise” –...
Read article

December 23, 2021
Year-End Roundup: Your Favorite Blog Posts of 2021
Cytel blogs bring you debate and discussion of the newest trends in statistics and quantitative strategy. In 2021, our...
Read article

December 20, 2021
Approaches in Adaptive Group Sequential Clinical Trials
The promising zone design is an adaptive design which allows for sample size re-estimation based on the results of an...
Read article

December 14, 2021
Is Promising Zone Design Optimal?
In traditional clinical trial design, the sample size is often determined to detect the target treatment effect with...
Read article

December 8, 2021
Conditional Powers Vs Bayesian Predictive Power for Adaptive Sample Size Reassessment
Despite the debate in the scientific community on adaptive sample size reassessment (SSR), noteworthy developments have...
Read article
November 30, 2021
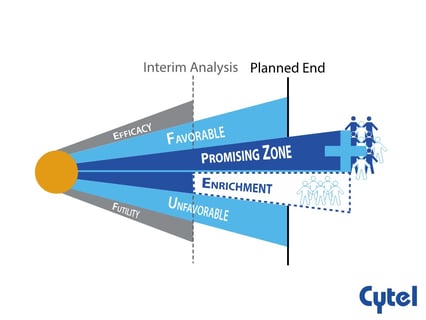
Dr. Julia Edwards Findings Promising Zone Designs
The aim of any clinical research is to detect the actual difference in treatment effect between two groups (power) and...
Read article

November 15, 2021
Optimal Promising Zone Designs: What Biotechs Need to Know
Since its first publication ten years ago, Cyrus Mehta and Stuart Pocock’s Promising Zone Design for sample size...
Read article

November 8, 2021
Keeping the Promise: Ten Year Anniversary of the Promising Zone Design
Ten years ago Cytel co-founder Professor Cyrus Mehta and Professor Stuart Pocock of the London School of Hygiene and...
Read article

June 30, 2021
Mathematical Methods for Clinical Trial Financial Strategy
When Cyrus Mehta introduced the Promising Zone Design over a decade ago, the new statistical method not only...
Read article

June 15, 2021
The Promising Zone Ten Years Later
Ten years ago, a seminal paper published by Cytel Founder Cyrus Mehta, introduced the Promising Zone Design to...
Read article

February 8, 2019
Publication Reveals New Promise for Promising Zone Designs
A 2018 publication in the Biometrical Journal by Cytel’s Cyrus Mehta, Lingyun Liu and Sam Hsiao, ‘Optimal Promising...
Read article

August 17, 2016
Adaptive Designs: In Conversation with the NEJM
Following the recent publication of their review article Adaptive Designs for Clinical Trials in the New England...
Read article
June 26, 2015
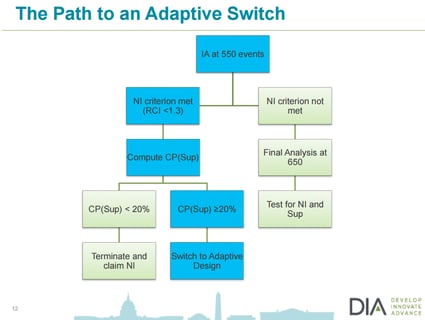
Clinical Trials: Why You Should Not Power for Superiority Upfront
Powering a trial for superiority can be financially risky. In some instances it may also prove unnecessary.
Read article
March 26, 2015
Statistical and Operational Challenges of the VALOR Trial
Last year Sunesis completed the VALOR trial, the first clinical study to make use of the groundbreaking promising zone...
Read article
February 10, 2015
How to Shorten a Cardiovascular Outcome Trial By Two Years
Cardiovascular outcome trials (CVOTs) have earned the reputation of being the untamable behemoths of the clinical...
Read article
September 18, 2014
5 times ‘Keep it Simple’ May Be Bad Advice for Clinical Designers
When designing clinical trials, many trial designers are advised to keep the trial simple. Prima facie, the keep it...
Read article
September 2, 2014
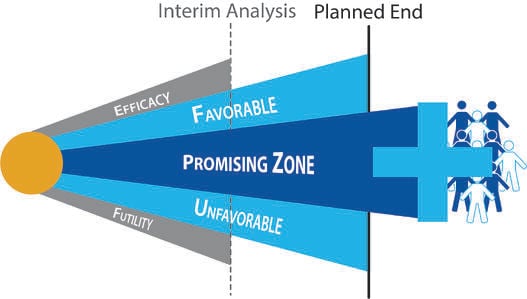
Impact of Study Design and Development Strategy on Pharmaceutical Programs and Portfolios
As more clinical trials make use of adaptive designs, investors have come to realize that high quality trial designs...
Read article

May 13, 2014
De-Risking Drug Development using Adaptive Design
The VALOR trial recently applied a promising zone design to a Phase 3 evaluation of Vosaroxin, a candidate for the...
Read article
May 5, 2014
The Perils of Poor Recruitment
A new JAMA study on discontinued randomized trials in Switzerland, Germany and Canada, reports that poor recruitment...
Read article


