Early Planning Strategies for External Control Arms in HTA and Regulatory Submissions

Written by Grace Hsu, Evie Merinopoulou, and Jason Simeone
To establish treatment efficacy and safety, regulatory and reimbursement decision-makers have traditionally preferred evidence from randomized clinical trials, which, by design, have a low risk of bias. However, single-arm trials (SAT) using an external control arm (ECA) are commonly performed for ethical reasons, due to the difficulties in identifying a suitable comparator arm(s) for head-to-head trials in a rapidly evolving therapeutic landscape and in recruiting patients in the case of rare diseases.
What are external control arms?
In clinical trials, the gold standard for evaluating a new treatment's effectiveness is a randomized controlled trial (RCT) with a comparator arm receiving a placebo or standard-of-care treatment. However, there are situations where RCTs might not be feasible.
External control arms, or synthetic control arms (SCAs), refer to the use of external data sources as a control group for comparison with a clinical study arm. Instead of the traditional placebo or active control group within the trial itself, researchers may use other data sources.
ECAs can be constructed from various sources, including:
- Historical trials: Data from previous RCTs that studied the comparator of interest.
- Real-world data: Previous real-world studies, observational data collected from electronic health records, disease registries, or claims databases.
When are external control arms used and what are acceptable use cases?
ECAs are used in situations where traditional control arms may be challenging or unethical to implement. Some acceptable use cases include:
- Rare diseases: When the patient population is small, recruiting enough participants for a traditional RCT can be challenging. This often leads to SATs being performed instead. ECAs offer a way to assess treatment effectiveness or other outcomes of interest compared to the existing options.
- Ethical considerations: In addition to rare disease scenarios, cases where withholding a potentially beneficial treatment from a control group would be unethical, such as in certain life-threatening conditions. ECAs allow for evaluation of a treatment relative to comparators without compromising patient well-being.
- Time and cost efficiency: Utilizing ECAs can sometimes streamline trial timelines and reduce costs associated with recruiting and managing control groups. Gathering early evidence for later trial planning can be done more effectively via ECAs.
- Multiple comparators: When the standard-of-care is unclear, potentially due to the use of different standards across countries or the existence of too many potential comparators, an RCT cannot be reasonably conducted for every comparison of interest. ECAs allow for any comparison to be done as long as an external data source of sufficient quality exists.
Current regulatory guidance on external control arms
Several organizations, including regulatory and HTA bodies, provide guidance on the use of ECAs. Some key examples are:
- U.S. Food and Drug Administration (FDA)1: The FDA has provided guidance on the use of real-world evidence (RWE) in regulatory decisions. This includes considerations for using ECAs and the criteria for when they may be acceptable via documents on using single-arm trials and external control arm data, acknowledging their potential role in specific situations.
- European Medicines Agency (EMA)2: The EMA also offers guidance for non-randomized studies. This includes considerations for the quality and relevance of the data, as well as statistical methods for comparison.
- NICE (National Institute for Health and Care Excellence)3, CADTH (Canadian Agency for Drugs and Technologies in Health): NICE and CADTH provide guidance on the use of ECAs in its reviews. Both emphasize the importance of a robust justification for the use of an ECA, focusing on their relevance to the decision problem, the feasibility of using them in the specific context of the appraisal, and the potential impact on the results. Discussion of how the ECA was designed, how it reflects current practice or standards of care, and how it relates to the patient population of interest.
- ICER (Institute for Clinical and Economic Review)4: ICER, an independent organization in the U.S. that assesses the value of medical treatments, often reviews studies with ECAs. They require a thorough justification for the choice of ECAs, including discussions on how the ECA reflects current clinical practice and how it impacts the study outcomes.
Generally, regulatory bodies and HTA agencies recognize the limitations of RCTs and increasingly acknowledge the utility of ECAs. However, as guidance evolves, consideration of the challenges in using ECAs and how they may be overcome becomes the key for generating robust evidence.
Important design and analytical considerations for sponsors using external control arms
When planning to use ECAs in HTA and regulatory submissions, sponsors should consider several factors: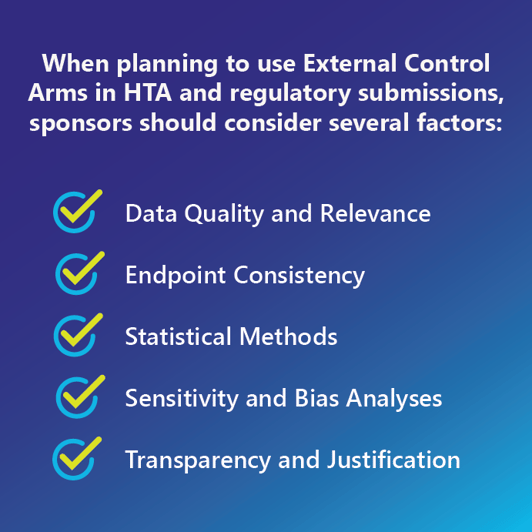
- Data quality and relevance: Researchers should ensure that the data used to construct the ECA is of high quality, relevant to the current study population, and ideally as similar as possible to the trial arm.
- Endpoint consistency: The endpoints used in an ECA study should be aligned between the external and trial cohorts to allow for meaningful comparisons. For example, progression may be measured differently in the real world than in a trial.
- Statistical methods: ECA studies involve choosing appropriate statistical methods to adjust for differences between the treatment group and the external control group owing to the lack of randomization. This may include propensity score matching, covariate adjustment, or other techniques. Planning for challenges specific for small sample sizes or other characteristics should be done.
- Sensitivity and bias analyses: This crucial element conducting sensitivity analyses to assess the robustness of the results to variations in assumptions or methods used with the external control data. Further, we expect bias from various sources, and their effect on study conclusions should be investigated.
- Real-world data often includes many challenges, such as missing data, measurement error, confounding due to unmeasured variables, etc. Analyses that quantitatively assesses how bias may change study conclusions provides decision-makers with the tools to evaluate evidence in a more nuanced manner even when derived from imperfect data sources.5
- Real-world data often includes many challenges, such as missing data, measurement error, confounding due to unmeasured variables, etc. Analyses that quantitatively assesses how bias may change study conclusions provides decision-makers with the tools to evaluate evidence in a more nuanced manner even when derived from imperfect data sources.5
- Transparency and justification: Sponsors should clearly explain their rationale for using ECAs and outline the methods employed to mitigate potential biases. In particular for the data source(s) used, justification of their selection as well as reasoning for not selecting excluded data sources should be well-documented.
Final takeaways
The use of ECAs is becoming an essential part of the evidence base for regulatory and reimbursement submissions. Successful acceptance of real-world ECAs relies on several factors including, but not limited to, the choice of an appropriate data source, appropriate methods for ensuring comparability between the trial and ECA populations, and accounting for uncertainty in the analyses due to data limitations/bias.
By understanding the role of ECAs and planning their use strategically, sponsors can navigate the evolving regulatory and HTA landscape. However, to fully satisfy the guidance given, and produce robust, regulatory-grade evidence from an ECA study, early planning strategies and considerations are crucial.
Interested in learning more?
Join Grace Hsu, Evie Merinopoulou, and Jason Simeone in Atlanta for ISPOR US, where they will be presenting “Ahead of the Curve: Navigating Early Planning Strategies for External Control Arms in HTA and Regulatory Submissions,” discussing ECA feasibility assessments, the optimal timing in the development process to plan for an ECA, how a sponsor can best prepare for early interactions to address differing regulatory and HTA perspectives, as well as other key elements for a successful submission.
Cytel’s Real-World and Advanced Analytics team will be at ISPOR US Booth #1018. Click to book a meeting with our experts:

 About Grace Hsu
About Grace Hsu
Grace Hsu is Director, Real-World Evidence, at Cytel. She has 9 years of experience in consulting and guiding project strategies. Grace holds a Master’s degree in Statistics, providing statistical consulting and strategy development for data curation, and the application of advanced analytics to clinical and RWD. Examples of her peer-reviewed publications include work on COVID-19, synthetic/external control arm comparative effectiveness analysis, quantitative bias analysis, Bayesian borrowing, and other methods of indirect comparison for both pharmaceutical research and HTA/regulatory submissions.

About Evie Merinopoulou
Evie Merinopoulou is Senior Director, Real-World Evidence, at Cytel. She is a health economist and real-world data scientist working on applications of real-world evidence in support of regulatory and HTA decision-making. She has worked in the healthcare consulting industry for over 10 years, currently leading the design and execution of observational research projects using global real-world data. Ms. Merinopoulou particularly focuses on projects involving real-world synthetic control arms, quantitative bias analysis, head-to-head comparisons using target trial emulation, and transportability analysis.

About Jason Simeone
Jason Simeone is Senior Director, Real-World Evidence, at Cytel. He is a pharmacoepidemiologist, with extensive experience in conducting studies to generate real-world evidence using U.S. claims and EMR data, as well as data sources globally. His research has focused on medication safety, effectiveness, burden of illness, and treatment patterns in a wide range of therapeutic areas, and he is particularly interested in the generation of real-world evidence for disease risk prediction and improvement of patient outcomes.
Notes
1 U.S. FDA. (2023). Considerations for the Design and Conduct of Externally Controlled Trials for Drug and Biological Products Guidance for Industry.
2 European Medicines Agency. (2023). Reflection paper on establishing efficacy based on single arm trials submitted as pivotal evidence in a marketing authorization.
3 National Institute for Health and Care Excellence. (2022). NICE real-world evidence framework.
4 Institute for Clinical and Economic Review. (2022). 2020-2023 Value Assessment Framework.
5 Thorlund, K., Duffield, S., Popat, S., Ramagopalan, S., Gupta, A., Hsu, G., Arora, P., & Subbiah, V. (2023). Quantitative bias analysis for external control arms using real-world data in clinical trials: A primer for clinical researchers. Journal of Comparative Effectiveness Research, 13(3), e230147.
Read more from Cytel's Perspectives:
Sorry no results please clear the filters and try again

FDA Guidance on the Design and Conduct of Externally Controlled Trials — What to Watch
The New EU HTA Landscape: Insights on Indirect Evidence

Key Elements and Implications of the Draft EU JCA Implementing Act


