Key Elements and Implications of the Draft EU JCA Implementing Act

Written by Lydia Vinals, PhD, and Grammati Sarri, PhD
The draft Implementing Act of the EU Health Technology Assessment Regulation (HTAR) for Joint Clinical Assessments (JCA) of medicinal products — the first legal definition of the procedural and methodological details for the new EU HTAR JCA — was published by the European Commission on March 5, 2024, following some delay.1 The draft is currently open for public consultation until April 2, and feedback received will be considered before the European Commission finalizes the Implementation Act.
Here, we delve into the key elements of the Implementing Act and the implications for sponsors.
Sneak peek into the future: Key elements of the draft Implementing Act
-
- Article 2
The Population, Intervention, Comparators, and Outcomes (PICO) scoping process central to the new EU HTAR is defined under Article 2. When submitting a marketing authorization application to the European Medicines Agency (EMA), sponsors will also provide information to the HTA Secretariat relevant to developing the assessment scope for the JCA of the medicinal product submitted. This information will come from the summary of the product characteristics and clinical overview section of the EMA submission. Notably, sponsors will only be asked for additional information in a meeting or in writing if deemed necessary. This suggests limited engagement in the PICO scoping process, with no apparent option for sponsors to submit PICO proposals or further clarification points in case of strong disagreement with the PICO final document.
-
- Article 12
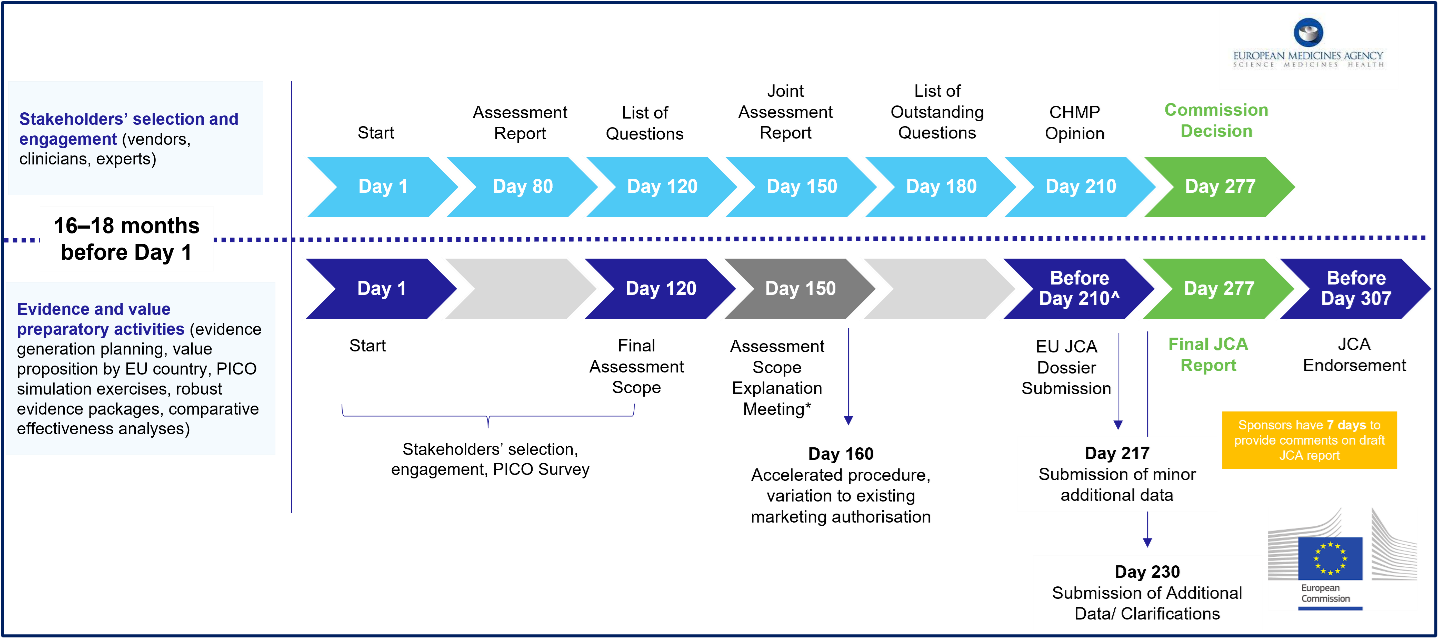
Article 12 confirms previously published tentative timelines. Sponsors will have 90 days from the HTA Secretariat finalizing the assessment scope to submit their JCA dossier. Figure 1 summarizes the EU HTAR JCA timelines (bottom) running parallel to the EMA submission process (top). At any point during the preparation of the draft joint clinical assessment and summary reports, sponsors will have seriously tight deadlines to respond to further requests — between 7 and 30 days to provide further information regarding the data submitted, data analyses, or additional evidence requested by the assessors.
Figure 1: Timelines and Preparatory Activities for EU JCA
-
- Article 16
Article 16 specifically covers potential changes in indications submitted to the EMA and highlights the potential for a new assessment scope being needed in these cases.
-
- Articles 6–10
Articles 6–10 outline external stakeholder engagement (e.g., clinical experts, patients, and relevant experts) in the JCA process. Notably, the HTA Secretariat can solicit input from external stakeholders at any stage while developing the assessment scope proposal.
The draft JCA dossier template, provided as an Annex to the Implementing Act, is heavily based on the structure and content of the dossier in the AMNOG framework in Germany.2 Of note, the cut-off date for the literature searches to identify the evidence to be included in the dossier has to be a maximum of 3 months before the submission. Also annexed to the Implementing Act is the template for the JCA report, which remains broad with limited detail.
Implications of the draft Implementing Act for sponsors
Overall, the draft JCA implementing underscores the following critical points for sponsors:
-
- Limited opportunity to provide input during the PICO scoping process
- Limited opportunity to provide input during the PICO scoping process
-
- Challenging timelines favoring the assessors
-
- Strong need for parallel preparatory activities to meet challenging timelines while creating broad evidentiary packages
-
- Build into strategic development a balance between the risk for additional (and perhaps unnecessary) preparatory activities versus agility to accommodate last-minute requests
-
- Consideration of the EU JCA timelines for local EU national submissions
However, there are several remaining unknowns, for example:
-
- What specific processes and methods will underpin the EU JCA?
- What specific processes and methods will underpin the EU JCA?
-
- How to address potential variation in additional local data requests?
-
- What will be the extent of detailed presentation of local data from the 27 EU member states within the EU JCA dossier?
-
- Will there be a potential benefit for sponsors to delay launching in local EU countries if the EU JCA report is not favorable? Should sponsors attempt to submit additional data, if possible?
Keeping these considerations in mind, how can sponsors best prepare for optimal JCA submissions? Early organizational preparation, stakeholder engagement and methodological readiness will be crucial for preparing timely and robust evidence packages, ensuring prompt EU JCA submissions and successful drug launches. In particular, sponsors should consider how to COLLABORATE via:
-
- Cross-functional training on EU HTAR and its implications
-
- Outlining global, regional (EU), and local evidentiary needs and methods acceptability criteria
-
- Learning from real-world data (RWD) including common data models and following EU-funded RWD case studies (e.g., DARWIN, SUSTAIN-HTA)
-
- Leveraging advanced indirect treatment comparison (ITC) methods (particularly in evidentiary challenging contexts such as reliance on single-arm trial data, surrogate endpoints, indications without a dedicated treatment pathway)3,4
-
- Anticipating stakeholder involvement for wider EU representation
-
- Building on patient involvement and consideration of additional value elements (e.g., equity)
-
- Outlining interdependencies between local and regional evidentiary needs for optimized submissions
-
- Recognizing commonalities, differences, and gaps in methodological guidance across EU markets
-
- Aligning EU JCA and local HTA evidence write-ups, where possible
-
- Timely updates on evidence packages and technology value story
-
- Evidence generation risk assessment
Interested in learning more? Lydia Vinals and Grammati Sarri will be hosting the webinar “From EU JCA to Local HTA Submissions: Understanding Commonalities, Differences, and Gaps in Methodological Guidance Across Key EU Markets” on Thursday, March 28, 2024, at 9 am ET. Can’t make the time? Register today and watch on-demand after the event:
 About Lydia Vinals
About Lydia Vinals
Lydia Vinals, PhD, is Associate Director and Senior Research Consultant at Cytel. She has over 6 years of experience successfully leading evidence synthesis projects ranging from early treatment landscape reviews and value frameworks to systematic literature reviews and comparative effectiveness research in support of health technology assessment submissions. She also has experience in leading health policy initiatives and has contributed to work on assessing the validity on non-randomized studies, which has been cited by the National Institute for Health and Care Excellence (NICE) real-world evidence framework. She is a core member of Cytel’s EU HTAR Task Force and supports clients in preparing for the upcoming Joint Clinical Assessment.
 About Grammati Sarri
About Grammati Sarri
Grammati Sarri, PhD, is Executive Research Principal at Cytel. She leads evidence generation strategies such as evidence synthesis (reviews, comparative effectiveness research), health policy efforts, and market access plans to support a successful product launch. With more than 15 years of experience in data synthesis, epidemiology, public health, and decision-making, Grammati provides strategic and subject matter support to Cytel clients to prepare for the new EU HTA Regulation and its requirements to enable successful local HTA applications for their products.
References
1 European Commission, “Health technology assessment — joint clinical assessments of medicinal products” [accessed March 7, 2024].
2 Gemeinsamer Bundesausschuss, “Formulare und Vorgaben zum Download – Anlagen zum 5. Kapitel der Verfahrensordnung” [accessed March 7, 2024].
3 Sarri, G., Rizzo, M., Upadhyaya, S., Paly, V. F., & Hernandez, L. (2024). Navigating the unknown: how to best 'reflect' standard of care in indications without a dedicated treatment pathway in health technology assessment submissions. Journal of comparative effectiveness research, 13(2), e230145.
4 Appiah, K., Rizzo, M., Sarri, G., & Hernandez, L. (2024). Justifying the source of external comparators in single-arm oncology health technology submissions: a review of NICE and PBAC assessments. Journal of comparative effectiveness research, 13(2), e230140.
Read more from Cytel Perspectives
Sorry no results please clear the filters and try again

Unravelling PICO: The Pillars of the European Joint Clinical Assessment

Real-Life Data-Sharing and EU Joint Clinical Assessments: Is Closing this Chasm a Mission Impossible?
Can RWE Help Restore Decades of Health Inequalities? Yes, and Here’s How


