
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

February 15, 2018
A recent publication in Biometrics ‘A Gatekeeping Procedure to Test a Primary and a Secondary Endpoint in a Group...
Read article

January 16, 2017
Adaptive Design Approaches from Cardiovascular Clinical Trialists Forum
The Global Cardiovascular Clinical Trialists Forum is a key event bringing together leading experts from across the...
Read article

August 17, 2016
Adaptive Designs: In Conversation with the NEJM
Following the recent publication of their review article Adaptive Designs for Clinical Trials in the New England...
Read article
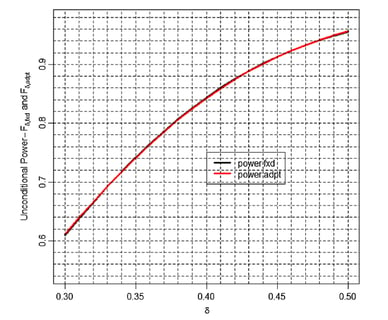
March 3, 2016
Adaptive SSR: Debunking the inefficiency myth
'The aim of a discussion should not be victory but progress.' This principle, expressed by the French essayist Joseph...
Read article
October 5, 2015
2 Methods for Evaluating Biomarker Subpopulations | Cytel
One consideration every sponsor of a biomarker-stratified confirmatory trial must take into account, is whether to...
Read article
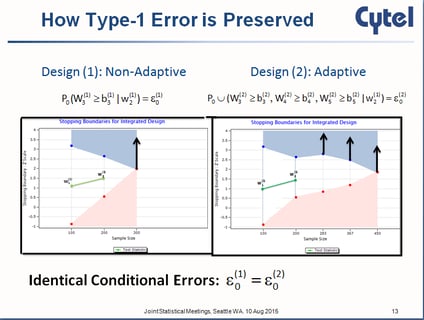
September 17, 2015
Inference on Confidence Intervals for Adaptive Designs: The Latest Breed of Adaptive Clinical Trials
Most people familiar with adaptive clinical trial designs are familiar with those statistical designs that reject the...
Read article
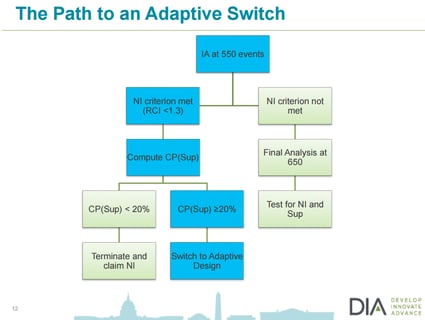
June 26, 2015
Clinical Trials: Why You Should Not Power for Superiority Upfront
Powering a trial for superiority can be financially risky. In some instances it may also prove unnecessary.
Read article
February 26, 2015
Successful Adaptive Confirmatory Dose-Response Pediatric Study
Cytel President and co-founder Cyrus Mehta has co-authored a paper on Infantile Hemangioma, recently published in the...
Read article
February 10, 2015
How to Shorten a Cardiovascular Outcome Trial By Two Years
Cardiovascular outcome trials (CVOTs) have earned the reputation of being the untamable behemoths of the clinical...
Read article
September 2, 2014
Impact of Study Design and Development Strategy on Pharmaceutical Programs and Portfolios
As more clinical trials make use of adaptive designs, investors have come to realize that high quality trial designs...
Read article
August 14, 2014
Backward Image Confidence Intervals for Adaptive Group Sequential Designs (Full Article Attached)
Cytel statisticians are looking foward to attending the Conference of the International Society for Clinical...
Read article

April 30, 2014
StatXact 25th Anniversary: Reflections of a Pioneer
For the second installment of our StatXact 25th Anniversary Retrospective Series, Professor Joan Hilton (UC San...
Read article

April 21, 2014
StatXact 25th Anniversary: A Horizon for the Stars
The core methodological problem that would eventually spur the development of Cytel’s StatXact software was first posed...
Read article


