
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

April 21, 2021
Two therapies are placed in head-to-head clinical trials when they are compared against each other as opposed to a...
Read article

September 4, 2020
Cytel Co-Founder Cyrus Mehta Presents at the Heart Failure Collaboratory, a Public-Private Partnership with FDA
On Friday September 11, Cyrus Mehta, co-founder of Cytel, will be delivering a talk to the Heart Failure Collaboratory,...
Read article

August 5, 2020
Design and Data Considerations from Cardiovascular Pilot Investigation
Cytel is conducting two pilot projects on head-to-head comparisons using real world data. These projects in oncology...
Read article

July 21, 2020
Head to Head Comparisons Using Real World Data
Cytel is conducting a webinar series that focuses on target trial emulation and causal inference approaches using real...
Read article
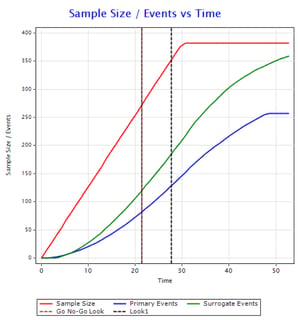
January 30, 2020
Designing Event-based Studies: Interview with Pantelis Vlachos
The Cytel Trial Design Innovations (CTDI) Webinar Series recently hosted a webinar on designing event-based studies....
Read article

January 16, 2020
Adaptive Population Enrichment in a Phase III Oncology Trial
January’s Cytel Trial Design Innovations (CTDI) Webinar Series will feature Biostatistician and pioneering Bayesian...
Read article
September 20, 2017
Accurate Event Prediction in a Cardiovascular Outcomes Research Trial
In this blog we share a case study of work our strategic consulting team conducted supporting accurate event prediction...
Read article

January 16, 2017
Adaptive Design Approaches from Cardiovascular Clinical Trialists Forum
The Global Cardiovascular Clinical Trialists Forum is a key event bringing together leading experts from across the...
Read article
October 13, 2015
Statistical Primer for Cardiovascular Research
In honor of World Obesity Day (celebrated on Oct. 11 2015) here is an American Heart Association Statistical Primer on...
Read article
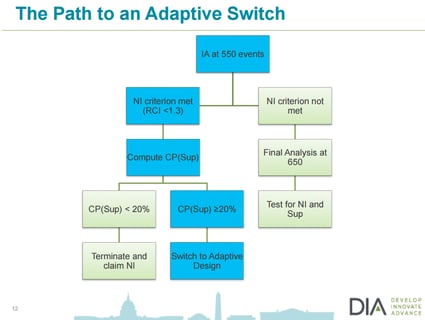
June 26, 2015
Clinical Trials: Why You Should Not Power for Superiority Upfront
Powering a trial for superiority can be financially risky. In some instances it may also prove unnecessary.
Read article
June 18, 2015
A Cautionary Tale about Composite Endpoint Construction: The ARISE Trial
In August 2006 AstraZeneca completed the ARISE trial, which aimed to determine whether AGI-1067 was effective in...
Read article
June 11, 2015
Aligning Clinical Development & Regulatory Objectives for Cardiovascular Outcome Trials
When the FDA first began to require pharmaceuticals to perform cardiovascular outcome trials to establish the safety of...
Read article
March 19, 2015
How to Plan Interim Looks in Adaptive Clinical Trials: 3 Strategies
A well-timed interim analysis can generally supply added benefits to the operational and administrative aspects of a...
Read article
February 10, 2015
How to Shorten a Cardiovascular Outcome Trial By Two Years
Cardiovascular outcome trials (CVOTs) have earned the reputation of being the untamable behemoths of the clinical...
Read article
February 3, 2015
Clinical Development & Statistical Methodology for Cardiovascular Risk Assessment
A new publication co-authored by Cytel Co-Founder and President Cyrus Mehta considers a range of clinical development...
Read article

October 21, 2014
Clinical Impact Beyond 'Time to First' Analyses
Every year, the East Users Group Meeting brings together notable experts from industry and academia to discuss the...
Read article


