Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.
November 4, 2014
A key stage of exploratory drug development is implementing a proof-of-concept study to demonstrate the safety of a...
Read article
September 18, 2014
5 times ‘Keep it Simple’ May Be Bad Advice for Clinical Designers
When designing clinical trials, many trial designers are advised to keep the trial simple. Prima facie, the keep it...
Read article
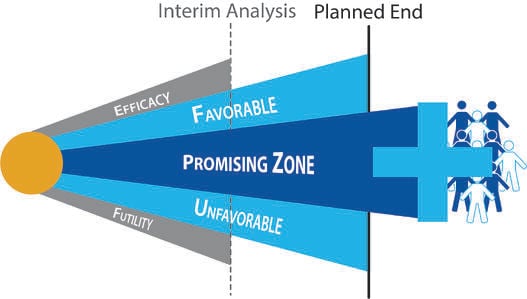
September 2, 2014
Impact of Study Design and Development Strategy on Pharmaceutical Programs and Portfolios
As more clinical trials make use of adaptive designs, investors have come to realize that high quality trial designs...
Read article


