
Cytel's blog featuring the latest industry insights.

Model-informed drug development has been defined by Richard Lalonde ( Lalonde, 2007) (1) as “Development and application of pharmaco-statistical models of drug efficacy and safety from preclinical and clinical data to improve drug development knowledge management and decision-making”. It has been identified by the FDA as an important way to help reduce attrition and uncertainty in drug development.
In a recent FDA Voice article,(2) FDA Commissioner Scott Gottlieb noted the critical role which modeling and simulation can play in making clinical development more efficient.
He commented that:
“FDA’s Center for Drug Evaluation and Research (CDER) is currently using modeling and simulation to predict clinical outcomes, inform clinical trial designs, support evidence of effectiveness, optimize dosing, predict product safety, and evaluate potential adverse event mechanisms. We’ll be putting out additional, updated guidance on how aspects of these in silico tools can be advanced and incorporated into different aspects of drug development.”
In this blog, we share a new Cytel infographic highlighting 4 key questions a sponsor can explore to apply these approaches within their development programs.
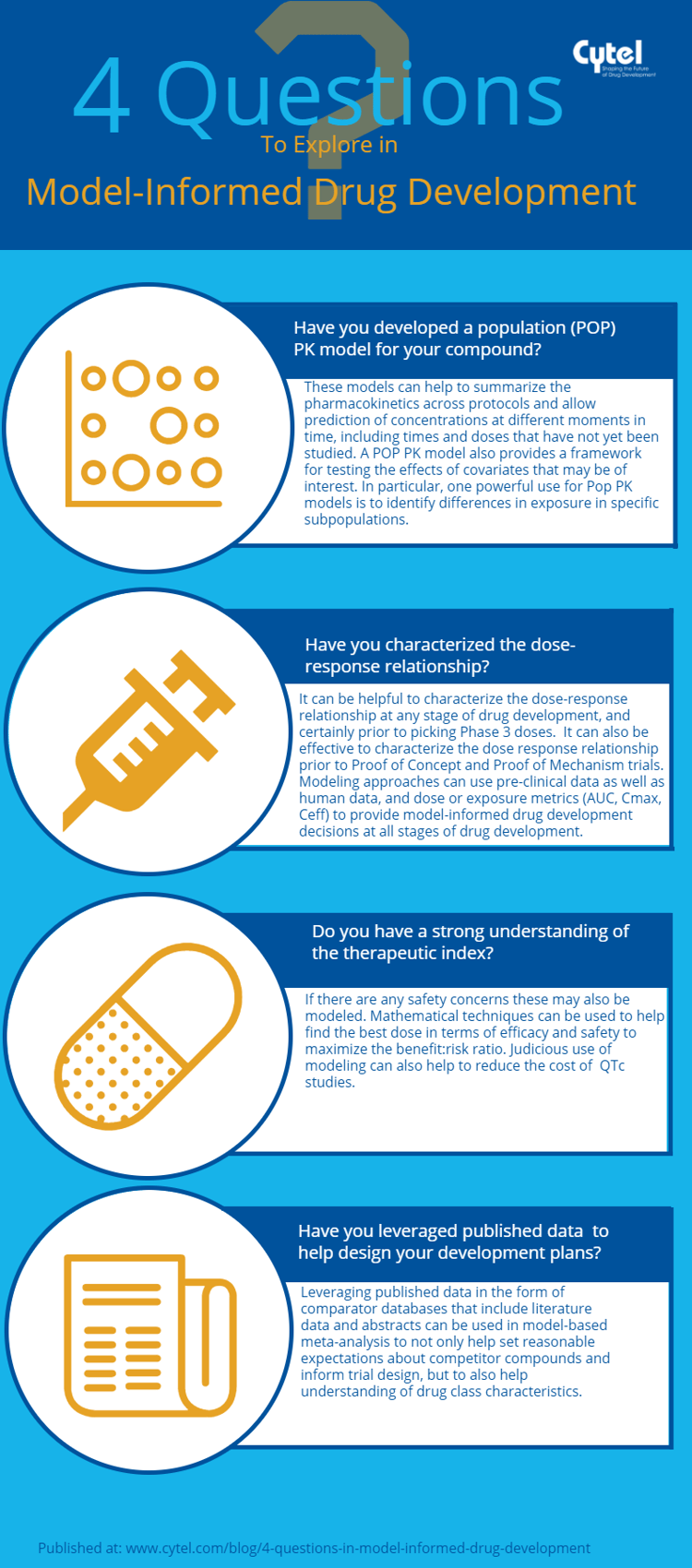
Click the button below to view the infographic in .pdf
References
2) How FDA Plans to Help Consumers Capitalize on Advances in Science | FDA Voice
Further reading
It's time to bridge the gap between pharmacometrics and biostatistics
An efficient tool for model-based meta-analysis
Case study: Dose response modeling in ulcerative colitis
To learn more about our Quantitative Pharmacology and Pharmacometrics Services click below:
