How do CDASH standards build data quality?


Data Standards play a crucial role in structuring and promoting long term value of clinical data.
Clinical Data Acquisitions Standards Harmonization or CDASH was developed with participation from all three ICH regions (US, Europe and Japan) with recommended data collection fields for 16 domains-> DEMOG, AE etc. It also includes implementation guidelines, best practice recommendations, and regulatory references. There is sometimes a misconception that CDASH defines the layout of the CRF and eCRF. This is not the case. The function of CDASH is to define the naming conventions for the clinical database, and outline how variables are mapped to SDTM. It defines how questions should be formulated for data collection within the CRF and eCRF making use of standard CDISC controlled terminology. In this blog, we will provide an example of CDASH implementation.
One key benefit of implementing CDASH standards is improvements to quality of outputs. With the development of more structured information, during study set up, design of eCRFs can become easier with ability to re-use and retrieve information. There can be a beneficial effect on timelines with time for tasks being considerably reduced. Beyond the immediate benefits during study set up, data review is improved through use of standards, and data transfers are eased. A standard approach across studies serves to support subsequent data analysis. Importantly, from a regulatory submission point of view, CDASH is is a starting point for ensuring we begin thinking about standards at the outset of the clinical data collection and analysis data flow, and supports traceability.
Below, we provide an example of implementation of CDASH within a CRF using fields that exist in CDASH. We’ll take the example of a vital signs page.
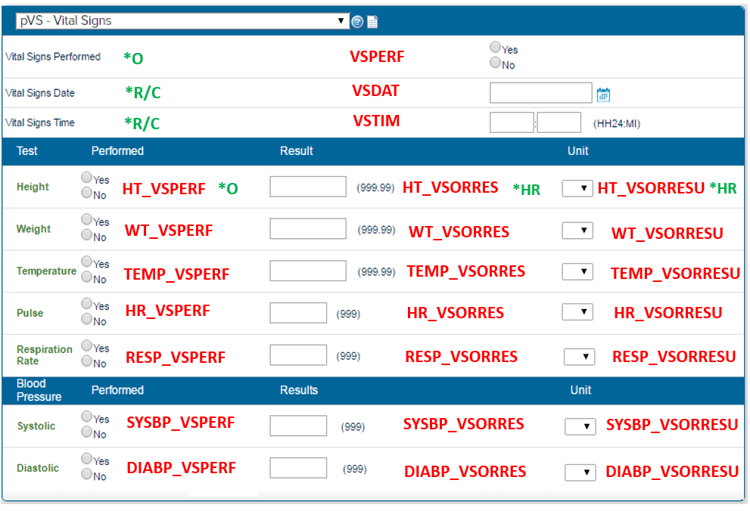
The CDASH core is a link connecting the variable name to its SDTM Core.
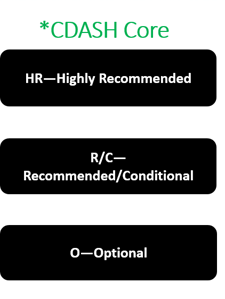
The letters in green are the CDASH Cores- each variable has a CDASH core attached to it.
HR—Highly Recommended; the field should be included on the CRF
R/C—Recommended/Conditional; the field should be collected on the CRF if certain conditions are met.
O—Optional; the field may be included on the CRF if it is needed to satisfy study requirements or for the sponsor’s operational database
A pattern is followed whereby the question Performed: Yes/No has been annotated to PERF, date to DAT, Time is annotated to TIM, Result is ORRES and Result unit annotated to ORRESU as standard.
The goal is to create a clear path from the variable name from the data capture system to the SDTM datasets and then onwards.
The close alignment between Cytel’s data management, statistical programming and biostatistics teams helps to ensure appropriate data lineage between CDASH , SDTM and onward to ADaM standards. Cytel has a track record of successful CDISC delivery and is a CDISC registered solutions provider.
This blog is based on aspects of Sanyogeeta Andhale and Makarand Deshmukh' s work for a recent presentation at the SCDM India in December. Many thanks to Sanyogeeta!
Liked this article? Get bonus content- download our new ebook Data Management Fundamentals for Your Next Clinical Trial


