History of Design Innovation

1987
2022

2022
Science of Trial Design

2021
Strides in Simulation-Guided Design (Solara), Bayesian Methods & Platform Trial

2020
Thought Leadership during COVID-19

2019
Next Generation of Advanced Quantitative Solutions
Cytel expands into real world data and real world evidence by aquiring Lighthouse and MTEK. This brings more basket and platform trials under its belt. This solidifies capabilities in historical borrowing, trial emulation, Bayesian modeling, single arm trials, natural history of disease, and other innovations. Cytel also makes its first foray into Global Health and epidemiology.
2018
Cardiovascular Health Takes Center Stage

2017
A New Chapter

2016
25 Years of Confirmatory Adaptive Designs
As a part of the global celebrations of 25 years of adaptive confirmatory designs, Cyrus Mehta publishes seminal paper reviewing the leading clinical trials with Adaptive Designs. The EXAMINE Trial, a trial for cardiovascular outcomes in patients with diabetes, was the first to change an endpoint as a part of the interim adaptation. Read more in the published results.

2015
Cytel Releases EnForeSys
Cytel releases Enforesys, which applies advanced knowledge of Monte Carlo methods to enrollment and feasibility forecasting. In doing so it presents a way to utilize simulations-based solutions to reduce enrollment uncertainty, which according to JAMA was the foremost reason for trial discontinuity at the time .
These simulations enabled sponsors to: forecast enrollment while accounting for variability at the site level; model fluctuations in enrollment rate for improved targeting; and build a more accurate, data-driven feasibility strategy with Monte Carlo modeling.
To learn more click here: https://www.cytel.com/whitepaper-enforesys
2010 - 2014
Making Complex Methods Accessible
In this era innovative trials became more widespread, available to biotechs for the first time. Cytel designs a number of trials including a promising zone design for the VALOR trial in relapsed/refractory Acute Myeloid Leukemia, and the endpoint adaptation for EXAMINE a trial for cardiovascular outcomes in patients with diabetes. Cytel also began to build the bridge between design and strategy with key publications in Bayesian decision-making.
East(R) releases a number of modules to empower statisticians to create complex, innovative and novel designs. This includes East Enrichment to create population enrichment designs for biomarker driven trials; as well as East Escalate for improved dose-finding; and Multi-arm Multi-stage trials that facilitate seamless Phase II/III designs.
2005 - 2010
Second Wave of Adaptive Designs
The second wave of adaptive designs to take shape in the industry included sample size re-estimation designs, enrichment designs, seamless phase designs, and more. Adaptations began to include changes to endpoint, changes to the number of trial arms carried forward, and the earliest attempts to tie design to decision sciences.
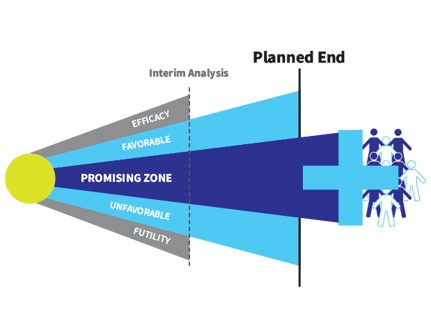
This era also included the development of the Promising Zone design. Invented by founder Cyrus Mehta and colleagues, this simple design ensures that new medicines do not fail to receive regulatory approval simply because the study is underpowered.
1995 - 2005
Cytel Becomes Leader in Adaptive & Group Sequential Designs as well as Biometrics Solutions
Cytel consolidates its position in adaptive and group sequential trials with dozens of publications. It also expands its biometrics services with offices in South Asia (Pune & Hyderabad), Switzerland (Geneva), and Western Europe (Paris, Barcelona and Lyon).

1990 - 1995
Cytel Software Becomes Industry Leader
In the early 1990s, Cytel's thought leadership focused on making small sample designs widely available in industry to expedite clinical trials. Wider access to software meant products like East, StatXact and LogXact hit the market and created the first generation of technology-enabled clinical trials.
At the same time, Cytel's scientists and statisticians continued to publish in innovative methodologies including Bayesian, Group Sequential and exact small sample methods.

1980s
Founding and Early Days
Cytel is founded in 1987 with an SBIR grant to bring StatXact to market. StatXact continues to be used for calculations of small samples, and is now the most expansive toolkit for small sample methods available in the industry.
StatXact came to market in 1989 (click to learn more about the story of early Cytel).

1980s (Pre-Founding)
Small Sample Methods
Contact Us
Fill in the form below to get in touch

