
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

May 15, 2015
Imagine that it’s been three years since the completion of a trial, and that suddenly a regulatory body calls into...
Read article
November 6, 2014
Translational Statistics: How to Move Beyond the Comfort Zone
Professor LJ Wei holds that rules are for lawyers, not (necessarily) clinicians. When designing modern clinical trials,...
Read article
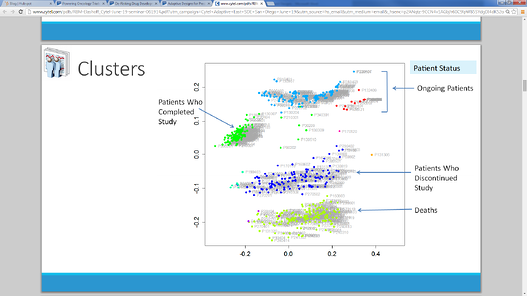
July 1, 2014
'Multivariate Approaches for Risk-Based Monitoring' An Adaptive Design (Slides Attached)
A recent Cytel Seminar on Adaptive Statistical Designs featured a talk by Michael Elashoff (Patient Profiles) on...
Read article

May 19, 2014
Frequentist? Time for an update!
Pantelis Vlachos, PhD, is a Director at Cytel Consulting. He works with a team of experts who regularly assist clinical...
Read article


