
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

July 27, 2020
Cytel is conducting a webinar series on complex innovative trial designs. Dr. Thomas Burnett, Senior Research Associate...
Read article

July 13, 2020
Interview with Dr. Thomas Burnett on Adaptive Enrichment
Cytel is hosting a complimentary webinar series that introduces biostatisticians and other members of the development...
Read article
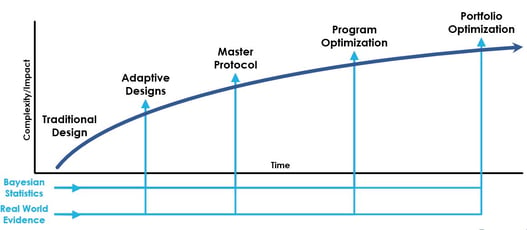
June 1, 2020
Webinar Replay: Innovative Drug Development at a Glance
In a recent interview with Cytel, Zoran Antonijevic, longstanding chair and leader of the DIA Adaptive Design...
Read article

May 18, 2020
Interview with Zoran Antonijevic on Adaptive Design Methods
In this blog, we speak with Zoran Antonijevic, longstanding chair and leader of the DIA Adaptive Design Scientific...
Read article

May 10, 2016
Subgroup Analyses in Early Phase Clinical Trials
We were fortunate to welcome Björn Bornkamp of Novartis to the EUGM 2016 presenting work he has developed jointly with...
Read article


