
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

July 27, 2021
Program and portfolio optimization creates a framework throughout the course of the clinical development journey, that...
Read article

December 2, 2020
Program and Portfolio Optimization: A New Paradigm
Significant advances have been made to enhance the efficiency of clinical trial designs. However, the traditional...
Read article

September 9, 2020
Importance of Designing Clinical Trials from a Program Perspective
Cytel’s co-founder, Nitin Patel, conducted a webinar on designing clinical trials from a program-level perspective. His...
Read article

August 25, 2020
Nitin Patel on Designing Clinical Trials from a Program Perspective
It is important to take a strategic approach to clinical development in order to minimize the potential for Phase 3...
Read article

November 12, 2018
Can Statisticians Contribute to Enhance the Position of Patients in Clinical Trials?
In this blog, we talk with Robert Greene, Founder and President of the HungerNDThirst Foundation, about his upcoming...
Read article

October 5, 2018
Interview with Stephen Senn: 70 Years and Still Here: The Randomized Clinical Trial and its Critics
We are delighted that Stephen Senn will be joining us at the EUGM on November 14th and 15th in Darmstadt, Germany. In...
Read article

September 27, 2018
Decision Making in Development Programs with Targeted Therapies: with Heiko Götte
In this blog, we talk with Heiko Götte, Senior Expert Biostatistician at Merck about his upcoming presentation at...
Read article

August 15, 2018
2018 East User Group Meeting Addresses Multiplicity Themes, with keynotes including Stephen Senn and Meinhard Keiser.
Cytel’s 7th East User Group Meeting (EUGM) will take place on November 14 & 15, 2018 at Merck in Darmstadt, Germany,...
Read article

July 3, 2018
Unveiling New East 6.5 Modules: Join Our Webinar
It’s shaping up to be a busy year for Cytel’s software development team with a number of upgrades and planned launches...
Read article

April 3, 2018
Maximizing Preclinical Knowledge for Optimal R&D
By Esha Senchaudhuri In response to its R&D productivity from 2005 – 2010, AstraZeneca took the initiative in 2011 to...
Read article

October 18, 2017
Webinar Replay: Phase 2 Trial Designs using Program-level Simulations
Cytel's new Trial Innovations Webinar Series provides a platform for the most promising new statistical approaches...
Read article
April 5, 2016
What's the price of pharma innovation?
Cost of pharmaceutical development and R&D productivity is an ongoing industry concern, consistently discussed in the...
Read article
February 10, 2015
How to Shorten a Cardiovascular Outcome Trial By Two Years
Cardiovascular outcome trials (CVOTs) have earned the reputation of being the untamable behemoths of the clinical...
Read article
September 18, 2014
5 times ‘Keep it Simple’ May Be Bad Advice for Clinical Designers
When designing clinical trials, many trial designers are advised to keep the trial simple. Prima facie, the keep it...
Read article
September 2, 2014
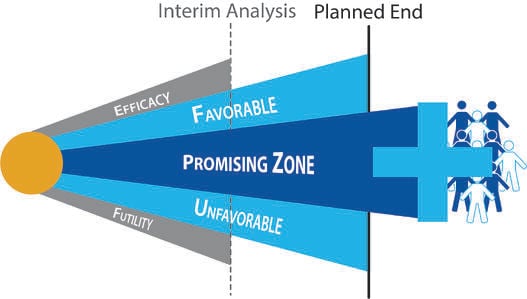
Impact of Study Design and Development Strategy on Pharmaceutical Programs and Portfolios
As more clinical trials make use of adaptive designs, investors have come to realize that high quality trial designs...
Read article


