Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

February 17, 2021
The COVID-19 Pandemic prompted the rapid surge in the generation of clinical data that has been scattered across...
Read article
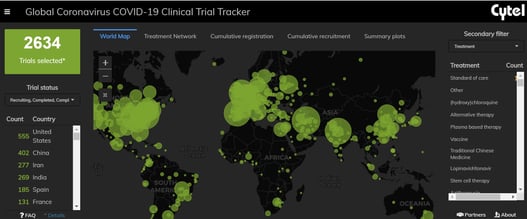
February 1, 2021
February 2021: Updates from the CYTEL COVID-19 Trial Tracker
Cytel’s COVID-19 Trial Tracker continues to provide real time updates to the status of COVID-19 clinical trials...
Read article

January 19, 2021
Cytel COVID Panel: Long-term Changes to Clinical Trials Due to the Pandemic
As we enter 2021 with new COVID-19 vaccines and greater optimism about the pipeline of drugs and devices positioned for...
Read article
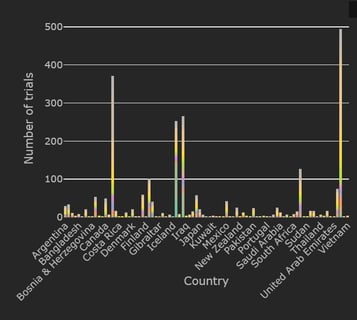
January 11, 2021
COVID-19 Trial Tracker Updates (January 11)
In April 2020, Cytel launched an open-access global COVID-19 Clinical Trial Tracker to help facilitate greater...
Read article

December 22, 2020
Year-end Roundup: Cytel’s Contributions Towards Health & Education in 2020
At Cytel, we have been diligently working to become an organization deeply committed to uplifting and enriching...
Read article
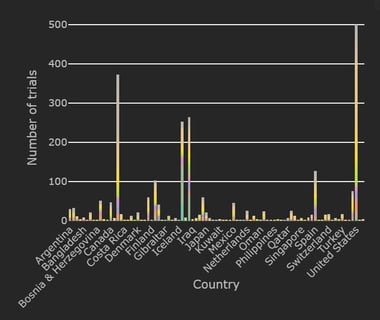
December 8, 2020
COVID-19 Trial Tracker Updates (December 8)
The Cytel COVID-19 Trial Tracker brings you an up to the minute, real time dashboard about COVID-19 trials around the...
Read article
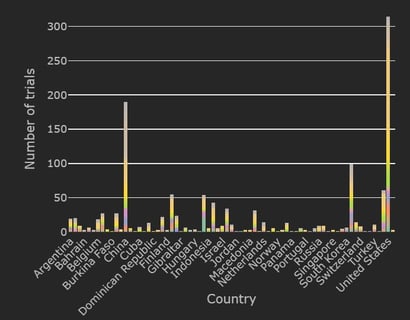
December 1, 2020
Mapping the Landscape of COVID-19 Clinical Trials in the US
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread. As...
Read article

June 9, 2020
Melinda Gates, on COVID-19 and Drug Development in Emerging Economies
Trevor Mundel leads the Bill & Melinda Gates Foundation’s efforts to develop high-impact interventions against the...
Read article
May 29, 2020
Trial Tracker: State of COVID-19 Development (May 2020)
At the close of May 2020, we have about 500 new trials globally but trends in trial design and choice of therapies...
Read article
May 22, 2020
4 Things You Need to Know about COVID-19 Trial Designs
The Cytel Trial Tracker now features summary plots that display trials by country, trial status and study design. This...
Read article
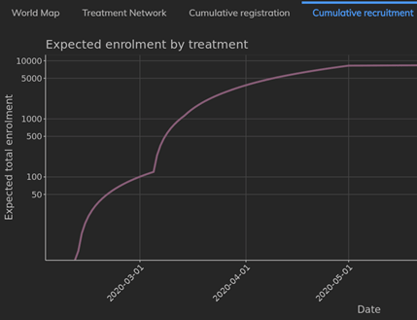
May 7, 2020
Weekly Insights from the COVID-19 Trial Tracker: Oxford Vacine Study
There are now over 950 trials registered, which means that 250 new trials were registered in the past week. Only 540 of...
Read article

May 7, 2020
COVID-19: Trials, Designs and Tools for Promising Results - A Virtual Panel Discussion
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread...
Read article


