
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

April 5, 2022
Hello! I’m delighted to pen my inaugural blog post here as Cytel’s Chief Medical Officer. In this series, we’ll explore...
Read article
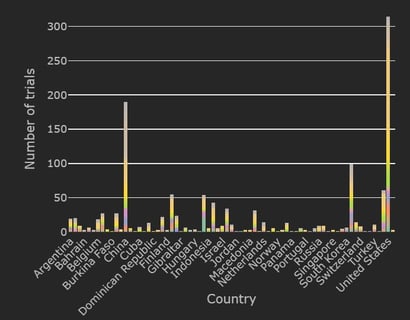
December 8, 2020
COVID-19 Trial Tracker Updates (December 8)
The Cytel COVID-19 Trial Tracker brings you an up to the minute, real time dashboard about COVID-19 trials around the...
Read article
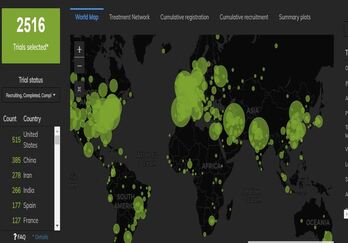
December 1, 2020
Mapping the Landscape of COVID-19 Clinical Trials in the US
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread. As...
Read article

May 7, 2020
COVID-19: Trials, Designs and Tools for Promising Results - A Virtual Panel Discussion
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread...
Read article

December 5, 2019
Biotechs and Medtechs, don’t forget your market access strategy (part 4 of 4): How to optimize your market access planning approach
Author: Michael S. Paas, Market Access & Commercialization Expert, Executive at AbbVie and Guest Author at Cytel In...
Read article

December 2, 2019
Biotechs and Medtechs, don’t forget your market access strategy (part 3 of 4): Harnessing the value of market access planning
Author: Michael S. Paas, Market Access & Commercialization Expert, Executive at AbbVie and Guest Author at Cytel...
Read article


