
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

January 19, 2022
The past two years have been transformative for Cytel. Most notably, the global COVID-19 pandemic unleashed an...
Read article

November 16, 2020
Bayesian Methods for Multiple Cohort Expansion (MuCE) designs
MUCE is a Bayesian solution for cohort expansion trials where multiple dose(s) and multiple indication(s) are tested in...
Read article
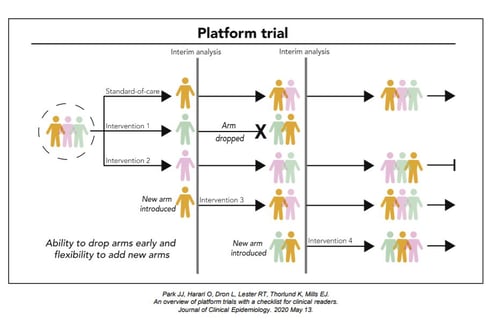
November 10, 2020
Key Design Considerations for Platform Trials
Platform trials are a new type of clinical trials where multiple interventions can be evaluated simultaneously against...
Read article

January 26, 2018
6 Innovative Trial Design Videos
The Cytel YouTube Channel hosts a wealth of video presentations from Cytel experts as well as external industry and...
Read article

March 29, 2017
New Publication: Design and Monitoring of Multi-Arm Multi-Stage Clinical Trials
With an increasing interest in platform designs and other innovative designs that involve multiple comparisons over...
Read article

September 29, 2016
Case studies:Learning from less-well understood adaptive designs
A paper "Best practices case studies for 'less well-understood' Adaptive designs", has been published by the DIA...
Read article

May 31, 2016
EAST 6.4 Release: Interview with Yannis Jemiai
Last week, we were delighted to announce the release of East 6.4 bringing further cutting –edge approaches to the East...
Read article

March 11, 2016
EAST takes on Multi-Arm Multi-Stage Designs
There has been increasing interest in multi-arm multi-stage trials with treatment selection and sample size...
Read article
March 27, 2015
Leveraging the Flexibility of an Adaptive Clinical Trials: A Case Study
We have often said that one of the greatest benefits of an adaptive clinical trial is the flexibility it affords for...
Read article
March 10, 2015
Embracing the Adaptive Mindset
Most of us are primed to think about the design of adaptive clinical trials as a narrow set of techniques applied to a...
Read article


