
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

September 15, 2020
In clinical trials with small or sparse data, statistical methods meant for large sample sizes may not be helpful to...
Read article

September 18, 2017
How to handle conservativeness of Exact p-value?
By Ashwini Joshi For small sample data or rare events data, exact non-parametric tests perform better than asymptotic...
Read article
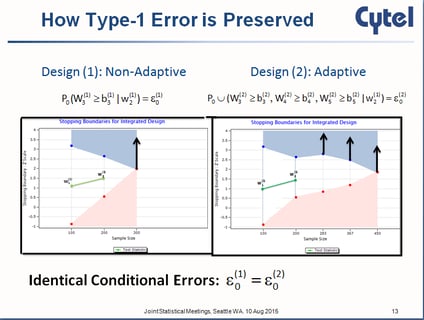
September 17, 2015
Inference on Confidence Intervals for Adaptive Designs: The Latest Breed of Adaptive Clinical Trials
Most people familiar with adaptive clinical trial designs are familiar with those statistical designs that reject the...
Read article

April 30, 2014
StatXact 25th Anniversary: Reflections of a Pioneer
For the second installment of our StatXact 25th Anniversary Retrospective Series, Professor Joan Hilton (UC San...
Read article

April 21, 2014
StatXact 25th Anniversary: A Horizon for the Stars
The core methodological problem that would eventually spur the development of Cytel’s StatXact software was first posed...
Read article


