
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

September 29, 2015
The Head of the DIA’s Adaptive Design Working Group Asks Us to Consider 5 ‘Soft-Skills’ All Effective Statisticians...
Read article
December 16, 2014
Cultivating Versatility in Statistical Consultants
Richard Branson once wrote: “I have always valued capability over expertise. While you may need to hire specialists for...
Read article
December 2, 2014
How to Incorporate New Technology into Your Clinical Development Strategy
During a recent DIA webinar on reinventing the clinical trial, Laurie Halloran (President of the Halloran Consulting...
Read article
November 4, 2014
New Exploratory Trial Method Translates into Better Financial Strategy
A key stage of exploratory drug development is implementing a proof-of-concept study to demonstrate the safety of a...
Read article
September 25, 2014
Part II: The Philosophy Behind a Software Package
A few weeks ago, we posted a synopsis of an event held at ISCB Vienna in which statisticians from Cytel, SAS and Stata...
Read article
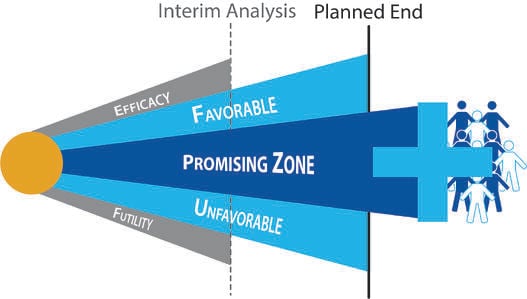
September 2, 2014
Impact of Study Design and Development Strategy on Pharmaceutical Programs and Portfolios
As more clinical trials make use of adaptive designs, investors have come to realize that high quality trial designs...
Read article

August 26, 2014
Statisticians from Cytel, SAS and Stata talk Software Development
During an invited speakers session at the lnternational Society for Clinical Biostatistics, Cytel VP Yannis Jemiai was...
Read article

August 12, 2014
What Horsepower Can Teach us about Well-Powered Trials
Beyond Wild Horses: Developing Innovation at Cytel "Horse-and-pony" by arjecahn on flickr. -...
Read article
August 5, 2014
Reflections on Statistical Entrepreneurship: An Interview with Nitin Patel
Cytel CTO Nitin Patel, recently sat down with ECHOES (a magazine for statistics in clinical trials) to discuss his...
Read article


