
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

December 2, 2020
Significant advances have been made to enhance the efficiency of clinical trial designs. However, the traditional...
Read article

August 19, 2020
Webinar on Adaptive Designs for Dose Finding: Part 2
Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson provided...
Read article

August 13, 2020
Webinar: Adaptive Designs for Dose Finding
Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson provided...
Read article
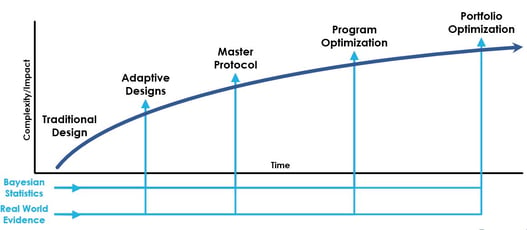
June 1, 2020
Webinar Replay: Innovative Drug Development at a Glance
In a recent interview with Cytel, Zoran Antonijevic, longstanding chair and leader of the DIA Adaptive Design...
Read article

May 18, 2020
Interview with Zoran Antonijevic on Adaptive Design Methods
In this blog, we speak with Zoran Antonijevic, longstanding chair and leader of the DIA Adaptive Design Scientific...
Read article

October 11, 2016
Simulations to optimize clinical trial programs
Its important to take a strategic approach to clinical development in order to minimize the potential for Phase 3...
Read article
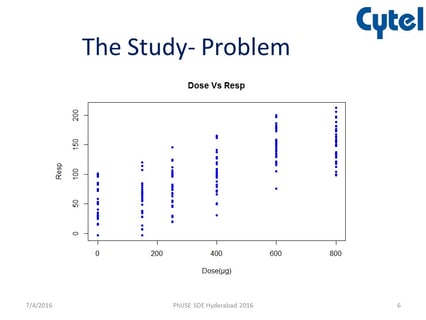
July 5, 2016
PROC MCPMod in Bronchodilator Case Study
At a recent PhUSE SDE, Cytel’s Chitra Tirodkar presented how East PROC MCPMod could be used to help solve the problem...
Read article

July 21, 2015
MCP-Mod for the Modern Dose-Ranging Clinical Trial
MCP-Mod methodology for dose-ranging clinical trials has been gaining popularity since the 2013 publication of the...
Read article


