
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

April 15, 2024
In the context of clinical trials, reducing the workload of the clinical team without compromising data quality is...
Read article

April 2, 2024
The FDA’s New Draft Guidance on DMCs: What to Know
Data monitoring committees (DMCs) review ongoing clinical trial data to make recommendations regarding trial conduct...
Read article

December 4, 2023
Preserving Trial Integrity After Receiving an Unanticipated IDMC Recommendation
Independent data monitoring committees review unblinded clinical trial data and issue recommendations to designated...
Read article

November 10, 2023
Conduct of IDMCs for Cell and Gene Therapy Trials
Independent data monitoring committees review unblinded clinical trial data and issue recommendations to designated...
Read article

September 25, 2023
Reducing Independent Data Monitoring Committee Timelines: A Focus on Formal Interim Analyses
As the pressure to reduce timelines rises across the industry, independent data monitoring committees (IDMCs) — which...
Read article

September 13, 2023
Setting Expectations for Formal Interim Analyses with Independent Data Monitoring Committees
Independent data monitoring committees (IDMCs) review ongoing clinical trial data to make recommendations regarding...
Read article

November 19, 2020
10 Key Qualifications for Independent Statisticians Reporting to the DMC
Data Monitoring Committees (DMCs) are groups of independent experts who periodically receive (by-arm) reports created...
Read article

November 27, 2019
Interview with David Kerr: Data Monitoring Committees (DMCs) – Behind Closed Doors
At the 2019 Challenges in Rare Diseases Clinical Trials Symposium and East training, Cytel partnered with Alexion to...
Read article
July 19, 2017
Case Study: Seamless Independent Data Monitoring Committee Support
With adaptive and innovative trial designs on the rise, operational implementation of interim analyses, including...
Read article

November 21, 2016
Infographic: 10 steps to consider before choosing an adaptive design
While adaptive designs can deliver significant benefits to clinical development- including ethical benefits for...
Read article

September 7, 2016
Overcoming challenges of 'Less Well Understood' Adaptive Designs
In the 2010 draft FDA ‘Guidance for Industry on Adaptive Design Clinical Trials for Drugs and Biologics', the agency...
Read article
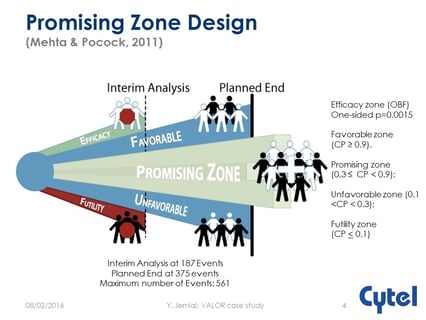
August 9, 2016
Operational and regulatory considerations in a promising zone trial
At the recent JSM Meeting, Cytel’s Yannis Jemiai presented the case study of the VALOR trial which used a promising...
Read article

June 16, 2016
Determining the future course of your trial
Predicting the course of a clinical trial is something which people will always want to do-whether for statistical...
Read article
June 14, 2016
Managing DMC analysis- an innovative programming solution
At Cytel, we are very often asked to get involved in DMCs ( Data Monitoring Committees) in a variety of capacities. Our...
Read article


