Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

July 27, 2018
In this blog, we share a new infographic based on this popular blog post illustrating some of the critical interactions...
Read article

April 25, 2018
Overcoming Data Management Challenges in Immuno-Oncology Trials
Data management is an essential building block for successful Immuno-Oncology (I-O) trials. At the Immuno-Oncology...
Read article

December 6, 2016
Ensuring quality data no matter the phase: data management considerations
The management of quality clinical data collection is built on a number of core essentials- including project...
Read article
August 2, 2016
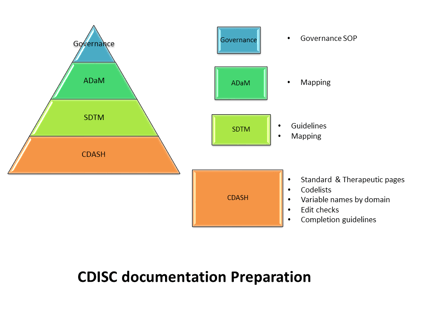
The CRO role in Data Standards Governance
Editor's note( this blog was refreshed in April 2018) As CDISC compliant submissions become increasingly expected,...
Read article
July 28, 2016
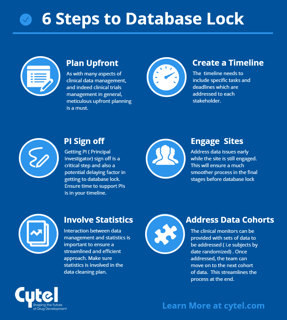
6 steps to timely database lock
To close a clinical database right the first time you have to begin with study start-up. Clearly, you can’t close a...
Read article

July 12, 2016
Key considerations in selecting an EDC system
How do you go about selecting the best Electronic Data Capture (EDC) system for your study? There is now a vast amount...
Read article

June 23, 2016
Getting the best out of your biometrics RFP
Vendor selection is a critical component of ensuring clinical trial success. A 2015 report (1) suggested that clinical...
Read article
April 26, 2016
Overcoming Data Management Challenges in Oncology Studies
In this blog we’ll highlight some unique challenges that are encountered from a Data Management perspective when...
Read article

April 20, 2016
Handling CDM data integrations
During the course of any clinical trial, there are often data which, while collected electronically, are outside of the...
Read article

April 15, 2016
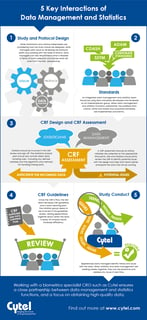
5 Key Interactions of Clinical DM and Statistics
It's critical for biostatistics and data management to be closely aligned and working effectively together. The...
Read article

February 26, 2016
Getting Technical: The evolving role of the Data Manager
Remember the early days of Electronic Data Capture? Those first systems, which were revolutionary for their time...
Read article


