
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

April 13, 2021
There has been an increasing use of digital measures in drug development recently. New wearables technologies can help...
Read article
April 2, 2021
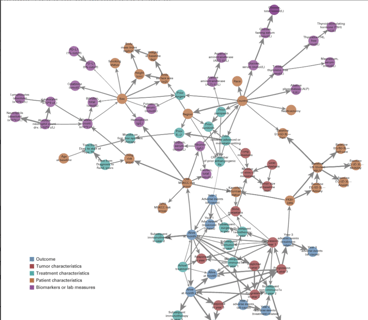
Using Bayesian Networks to Predict Survival Outcomes: New Case Study
Earlier this month, my colleagues at Cytel Canada published a paper in JCO Clinical Cancer Informatics, offering a...
Read article

March 31, 2021
Wearables and Decentralization
As decentralized clinical trials become more attractive in an era of COVID-19, the role of wearables in clinical...
Read article

March 11, 2021
Data and analysis in Modern Oncology Clinical Development
In the recent years, Oncology trials are seeing a technological shift that is expected to make them faster and more...
Read article

February 25, 2021
Use of Wearables in Confirmatory Clinical Trials
The convergence of several distinct trends has made wearables an increasingly attractive option for use in confirmatory...
Read article

February 11, 2021
The biostats and clinical overview of a growing clinical strategy
The past two years have witnessed a heightened interest in the use of wearables in clinical development. The unexpected...
Read article

February 9, 2021
New Meta-Analysis in JAMA Uses Novel Quantitative Techniques to Demonstrate Baseline Characteristics Informing Response to Common Therapy for Kidney Cancer
Recent years have witnessed improving survival outcomes for those struggling with a range of common kidney cancers....
Read article
April 29, 2020
Webinar: Transparent Machine Learning in Oncology
In our previous blog, we spoke with Alind Gupta, who works as a Machine Learning Researcher at Cytel in Canada. The...
Read article

April 20, 2020
Interview with Alind Gupta: Transparent Machine Learning in Oncology
Cytel is hosting a webinar on Transparent Machine Learning in Oncology, on April 21, 2020. Our speaker, Alind Gupta,...
Read article
March 31, 2020
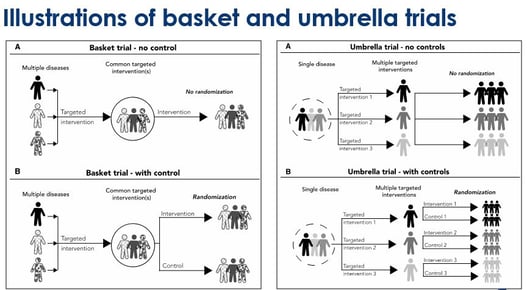
Key Design Thoughts for Basket Trials and Umbrella Trials by Jay Park
Since 1953, when the discovery of the structure of DNA was made, we have seen great advancements in genomics....
Read article

March 12, 2020
Interview with Jay Park: The present and future of Master Protocols
In September 2018, the FDA provided a draft guidance on master protocols reflecting an increased interest in these...
Read article


September 27, 2018
Decision Making in Development Programs with Targeted Therapies: with Heiko Götte
In this blog, we talk with Heiko Götte, Senior Expert Biostatistician at Merck about his upcoming presentation at...
Read article
November 1, 2016
Pharmacometrics tools of the trade: 4 factors to consider
Unlike statistics which has been around in some form for hundreds of years, pharmacometrics is, by comparison, a...
Read article
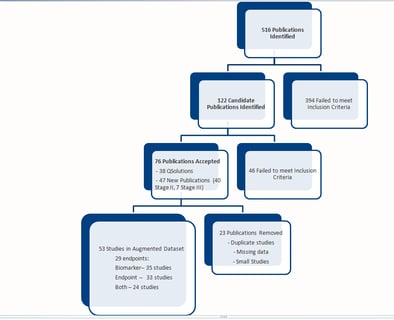
November 3, 2015
Pharmacometrics for Biomarker Driven Clinical Strategy
QPP (sometimes called QP2) remains at the heart of model based drug development. Short for Quantitative Pharmacology &...
Read article
October 5, 2015
2 Methods for Evaluating Biomarker Subpopulations | Cytel
One consideration every sponsor of a biomarker-stratified confirmatory trial must take into account, is whether to...
Read article

September 4, 2015
Using Simulation for Accelerated Early Phase Drug Development
Our Client's Challenge: Can knowledge of the relationship between biomarkers and clinical endpoints help us to optimize...
Read article


